Shop for Plans
Shop for your own coverage
Plans through your employer
Learn about the medical, dental, pharmacy, behavioral, and voluntary benefits your employer may offer.
Learn
Living or working abroad?
Childhood Craniopharyngioma Treatment (PDQ®): Treatment - Health Professional Information [NCI]
General Information About Childhood Craniopharyngioma
Primary brain tumors, including craniopharyngiomas, are a diverse group of diseases that together constitute the most common solid tumors of childhood. Brain tumors are classified according to an integrated assessment of histology and molecular characteristics, with tumor location and extent of spread as important factors that affect treatment and prognosis.
Craniopharyngiomas are uncommon pediatric brain tumors. They are believed to be congenital in origin, arising from ectodermal remnants, Rathke cleft, or other embryonal epithelium. They often occur in the suprasellar region with an intrasellar portion. Magnetic resonance imaging (MRI) and computed tomography (CT) imaging are used to diagnose craniopharyngiomas, but histological confirmation is generally required before treatment.
The treatment of patients with newly diagnosed craniopharyngiomas may include surgery, radiation therapy, cyst drainage, and intracystic therapies. The treatment of patients with recurrent craniopharyngiomas depends on the initial treatment used. With current treatment strategies, the 5-year and 10-year survival rates reach 80% to 90% for children between the ages of 0 and 14 years.[
The PDQ childhood brain tumor treatment summaries are organized primarily according to the World Health Organization Classification of Central Nervous System (CNS) Tumours.[
Incidence
Craniopharyngiomas are relatively uncommon, accounting for about 3% of all intracranial tumors in children.[
No predisposing factors have been identified.
Anatomy
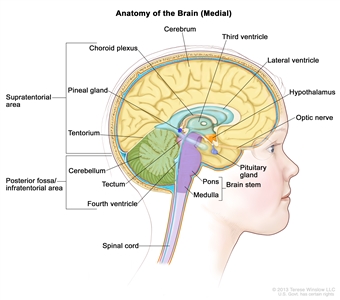
Figure 1. Anatomy of the inside of the brain, showing the pineal and pituitary glands, optic nerve, ventricles (with cerebrospinal fluid shown in blue), and other parts of the brain. The tentorium separates the cerebrum from the cerebellum. The infratentorium (posterior fossa) is the region below the tentorium that contains the brain stem, cerebellum, and fourth ventricle. The supratentorium is the region above the tentorium and denotes the region that contains the cerebrum.
Clinical Presentation
Craniopharyngiomas occur in the suprasellar region, near the pituitary gland, optic nerves, and optic chiasm (see
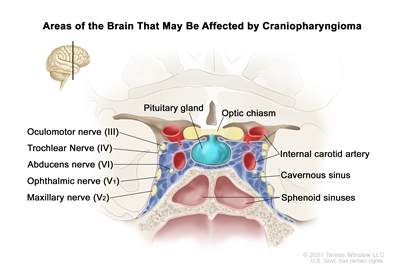
Figure 2. Drawing showing a coronal view of the inside of the brain where craniopharyngiomas may form. The tumor usually occurs in the region of the pituitary gland, near the optic chiasm and optic nerves.
Diagnostic Evaluation
CT and MRI scans are often diagnostic for childhood craniopharyngiomas, with most tumors demonstrating intratumoral calcifications and a solid and cystic component. MRI of the spinal axis is not routinely performed.
Craniopharyngiomas without calcification may be confused with other tumor types, including germ cell tumors, hypothalamic/chiasmatic astrocytomas, or Langerhans cell histiocytosis. Biopsy or resection is required to confirm the diagnosis.[
Apart from imaging, patients undergo endocrine testing and formal vision examination, including visual-field evaluation.
Prognosis
Regardless of the treatment modality, the 5-year and 10-year overall survival rates range from 80% to 90% in children between the ages of 0 and 14 years.[
References:
- Ostrom QT, Cioffi G, Waite K, et al.: CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol 23 (12 Suppl 2): iii1-iii105, 2021.
- Louis DN, Perry A, Wesseling P, et al.: The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23 (8): 1231-1251, 2021.
- WHO Classification of Tumours Editorial Board, ed.: WHO Classification of Tumours: Central Nervous System Tumours. Vol. 6. 5th ed. IARC Press; 2021.
- Karavitaki N, Wass JA: Craniopharyngiomas. Endocrinol Metab Clin North Am 37 (1): 173-93, ix-x, 2008.
- Garnett MR, Puget S, Grill J, et al.: Craniopharyngioma. Orphanet J Rare Dis 2: 18, 2007.
- van Schaik J, Hoving EW, Müller HL, et al.: Hypothalamic-Pituitary Outcome after Treatment for Childhood Craniopharyngioma. Front Horm Res 54: 47-57, 2021.
- Jimenez RB, Ahmed S, Johnson A, et al.: Proton Radiation Therapy for Pediatric Craniopharyngioma. Int J Radiat Oncol Biol Phys 110 (5): 1480-1487, 2021.
- Bogusz A, Müller HL: Childhood-onset craniopharyngioma: latest insights into pathology, diagnostics, treatment, and follow-up. Expert Rev Neurother 18 (10): 793-806, 2018.
- Tan TS, Patel L, Gopal-Kothandapani JS, et al.: The neuroendocrine sequelae of paediatric craniopharyngioma: a 40-year meta-data analysis of 185 cases from three UK centres. Eur J Endocrinol 176 (3): 359-369, 2017.
- Cohen M, Bartels U, Branson H, et al.: Trends in treatment and outcomes of pediatric craniopharyngioma, 1975-2011. Neuro Oncol 15 (6): 767-74, 2013.
- Fouda MA, Scott RM, Marcus KJ, et al.: Sixty years single institutional experience with pediatric craniopharyngioma: between the past and the future. Childs Nerv Syst 36 (2): 291-296, 2020.
- Nuijts MA, Veldhuis N, Stegeman I, et al.: Visual functions in children with craniopharyngioma at diagnosis: A systematic review. PLoS One 15 (10): e0240016, 2020.
- Wan MJ, Zapotocky M, Bouffet E, et al.: Long-term visual outcomes of craniopharyngioma in children. J Neurooncol 137 (3): 645-651, 2018.
- Ravindra VM, Okcu MF, Ruggieri L, et al.: Comparison of multimodal surgical and radiation treatment methods for pediatric craniopharyngioma: long-term analysis of progression-free survival and morbidity. J Neurosurg Pediatr 28 (2): 152-159, 2021.
- Felicetti F, Brignardello E, van Santen HM, eds.: Endocrine and Metabolic Late Effects in Cancer Survivors. Basel, Switzerland: Karger, 2021.
- Zhou L, Luo L, Xu J, et al.: Craniopharyngiomas in the posterior fossa: a rare subgroup, diagnosis, management and outcomes. J Neurol Neurosurg Psychiatry 80 (10): 1150-4, 2009.
- Rossi A, Cama A, Consales A, et al.: Neuroimaging of pediatric craniopharyngiomas: a pictorial essay. J Pediatr Endocrinol Metab 19 (Suppl 1): 299-319, 2006.
- Muller HL: Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm Res 69 (4): 193-202, 2008.
- Müller HL: Childhood craniopharyngioma--current concepts in diagnosis, therapy and follow-up. Nat Rev Endocrinol 6 (11): 609-18, 2010.
- Zacharia BE, Bruce SS, Goldstein H, et al.: Incidence, treatment and survival of patients with craniopharyngioma in the surveillance, epidemiology and end results program. Neuro Oncol 14 (8): 1070-8, 2012.
- Ostrom QT, Cioffi G, Gittleman H, et al.: CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol 21 (Suppl 5): v1-v100, 2019.
- Beckhaus J, Friedrich C, Boekhoff S, et al.: Outcome after pediatric craniopharyngioma: the role of age at diagnosis and hypothalamic damage. Eur J Endocrinol 188 (3): , 2023.
- Merchant TE, Dangda S, Hoehn ME, et al.: Pediatric Craniopharyngioma: The Effect of Visual Deficits and Hormone Deficiencies on Long-Term Cognitive Outcomes After Conformal Photon Radiation Therapy. Int J Radiat Oncol Biol Phys 115 (3): 581-591, 2023.
Histopathological Classification of Childhood Craniopharyngioma
Craniopharyngiomas are histologically benign and often occur in the suprasellar region, with an intrasellar portion. They may be locally invasive and typically do not metastasize to remote brain locations.
Craniopharyngiomas are classified under the category of tumors of the sella region according to the defined entities below. The two entities were previously described as subtypes of craniopharyngioma. However, based on the different populations they tend to affect, combined with distinct clinical, histological, and molecular characteristics, these are now considered unique diagnoses.[
- Adamantinomatous: Adamantinomatous craniopharyngiomas most frequently occur in children.[
2 ] These tumors are typically composed of a solid portion formed by nests and trabeculae of epithelial tumor cells, with an abundance of calcification, and a cystic component that is filled with a dark, oily fluid. Wet keratin is also characteristic of this tumor type. Adamantinomatous craniopharyngiomas are more locally aggressive than are papillary craniopharyngiomas and have a significantly higher rate of recurrence.[3 ] Activating CTNNB1 gene variants are found in most adamantinomatous tumors.[1 ,4 ,5 ,6 ] - Papillary: Papillary craniopharyngiomas occur primarily in adults. BRAF V600E variants are observed in nearly all papillary craniopharyngiomas.[
1 ,5 ,6 ]
References:
- Louis DN, Perry A, Wesseling P, et al.: The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23 (8): 1231-1251, 2021.
- Karavitaki N, Wass JA: Craniopharyngiomas. Endocrinol Metab Clin North Am 37 (1): 173-93, ix-x, 2008.
- Pekmezci M, Louie J, Gupta N, et al.: Clinicopathological characteristics of adamantinomatous and papillary craniopharyngiomas: University of California, San Francisco experience 1985-2005. Neurosurgery 67 (5): 1341-9; discussion 1349, 2010.
- Sekine S, Shibata T, Kokubu A, et al.: Craniopharyngiomas of adamantinomatous type harbor beta-catenin gene mutations. Am J Pathol 161 (6): 1997-2001, 2002.
- Brastianos PK, Taylor-Weiner A, Manley PE, et al.: Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet 46 (2): 161-5, 2014.
- Goschzik T, Gessi M, Dreschmann V, et al.: Genomic Alterations of Adamantinomatous and Papillary Craniopharyngioma. J Neuropathol Exp Neurol 76 (2): 126-134, 2017.
Treatment Option Overview for Childhood Craniopharyngioma
Treatments for pediatric craniopharyngiomas have traditionally included maximal safe surgical resection and radiation therapy to treat residual tumor. Additionally, intracystic therapies such as radioactive phosphorus P 32, bleomycin, and interferon-alpha have been used. Evidence has demonstrated that conservative surgical approaches lead to better neuroendocrine and quality-of-life outcomes in patients.[
| Treatment Group | Treatment Options |
|---|---|
| Newly diagnosed childhood craniopharyngioma | |
| |
|
| |
|
| |
|
| Progressive or recurrent childhood craniopharyngioma | |
| |
|
| |
|
| |
|
| |
References:
- Lohkamp LN, Kasper EM, Pousa AE, et al.: An update on multimodal management of craniopharyngioma in children. Front Oncol 13: 1149428, 2023.
- Bogusz A, Müller HL: Childhood-onset craniopharyngioma: latest insights into pathology, diagnostics, treatment, and follow-up. Expert Rev Neurother 18 (10): 793-806, 2018.
Treatment of Newly Diagnosed Childhood Craniopharyngioma
There is no consensus on the optimal treatment for patients with newly diagnosed craniopharyngioma, in part because of the lack of prospective randomized trials that compare the different treatment options. Treatment is individualized on the basis of the following factors:
- Tumor size.
- Tumor location.
- Extension of the tumor.
- Potential short-term and long-term toxicity, partially related to baseline neuroendocrine and vision deficits (i.e., more conservative surgical approaches may be prioritized in patients who do not have existing neuroendocrine or visual deficits to mitigate the risk of surgical morbidity).[
1 ]
Established treatment options for newly diagnosed childhood craniopharyngioma include the following:
-
Complete resection with or without radiation therapy . -
Subtotal resection with radiation therapy . -
Primary cyst drainage with or without radiation therapy . -
Intracystic therapy .
Complete Resection With or Without Radiation Therapy
It may be possible to remove all visible tumor and achieve long-term disease control.[
Many surgical approaches have been described, and the choice is determined by tumor size, location, extension, and the patient's baseline signs and symptoms of disease. Surgical approaches include the following:
- Craniotomy: As noted above, gross-total resection may be technically challenging because the tumor is surrounded by vital structures. The surgeon often has a limited view of the hypothalamic and sellar regions, and portions of the mass may remain after surgery, accounting for some recurrences. An understanding of the complex variations in how the tumors grow anatomically may help facilitate gross-total resection.[
10 ] Nonetheless, almost all craniopharyngiomas attach to the pituitary stalk. Of the patients who undergo complete resection, virtually all will require lifelong pituitary hormone replacement with multiple medications.[3 ,11 ] - Transsphenoidal approach: A transsphenoidal approach has been proven possible in patients of all ages and for tumors of various sizes localized within the sella.[
12 ]; [13 ][Level of evidence C1] The development of expanded endonasal techniques with endoscopic visualization has allowed increased use of this approach, even for sizeable childhood tumors, which is similar to the experience in adults.[14 ] A complete resection can be obtained using this approach, with associated complications of panhypopituitarism and the risk of cerebrospinal fluid leaks.[15 ,16 ] When an endonasal approach is not possible, a craniotomy is required.
Complications of complete resection using either approach include the following:
- Obesity, which can be life-threatening.[
17 ] - Need for hormone replacement therapy.[
18 ] - Severe behavioral problems.[
18 ] - Blindness.
- Seizures.
- Spinal fluid leak.
- False aneurysms.
- Difficulty with eye movements.
- Death from intraoperative hemorrhage, hypothalamic damage, or stroke (rare).
If the surgeon indicates that the tumor was not completely removed or if postoperative imaging reveals residual craniopharyngioma, radiation therapy may be recommended to prevent early progression.[
Routine surveillance using magnetic resonance imaging is performed for several years after complete resection because of the possibility of tumor recurrence.
Subtotal Resection With Radiation Therapy
The goal of limited surgery can be to establish a diagnosis, drain cystic components of the tumor, and decompress surrounding anatomical structures. In subtotal resections, removal of the tumor from the pituitary stalk or hypothalamus is typically avoided to minimize the late effects associated with complete resection.[
Surgery is often followed by radiation therapy, because radiation therapy can decrease the risk of recurrence after a subtotal resection.[
Surgical complications with a subtotal resection can be similar to, but are less likely than, with a complete resection. If radiation therapy is used, additional complications must be considered, including the following:
- Loss of pituitary hormonal function.
- Cognitive dysfunction.
- Development of late strokes and vascular malformations.
- Delayed blindness.
- Development of second tumors.
- Malignant transformation of the primary tumor within the radiation field (rare).[
39 ,40 ]
A phase II single-arm study included 94 patients (aged 12 months to 21 years) with craniopharyngiomas who were treated with proton-beam radiation therapy after individualized surgical resection. These patients were compared with a historical cohort of patients who were treated with photon-beam radiation therapy.[
A long-term study of 101 children who were treated for craniopharyngiomas evaluated visual, neurocognitive, and endocrine outcomes after photon radiation therapy. Race and presence of a shunt affected baseline scores.[
A report from the prospective registry study KiProReg examined the use of proton-beam therapy in 84 children younger than 18 years with craniopharyngioma.[
Primary Cyst Drainage With or Without Radiation Therapy
For predominantly cystic craniopharyngiomas, stereotactic drainage of the cyst, insertion of a catheter from which drainage can be facilitated, or cyst fenestration are other therapeutic alternatives.[
- Draining the cyst to relieve pressure and complicating symptoms.
- Resecting the tumor or employing radiation therapy later.
Intracystic Therapy
Intracystic therapies include peginterferon alpha, radioactive phosphorus P 32 (32P) or other compounds,[
A systematic review of publications on the treatment of cystic craniopharyngiomas with radioisotope brachytherapy from 2010 to 2021 identified 66 pediatric patients (N = 228).[
Treatment Options Under Clinical Evaluation
Information about NCI-supported clinical trials can be found on the
Preclinical contemporary evaluations have identified active molecular and immune pathways in craniopharyngioma that may be targetable using commercially available or investigational agents. Specifically, MAPK and RAF pathways and immune/inflammatory targets such as PD-1 pathway components and IL-6 have been identified.[
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT05465174 (Nivolumab and Tovorafenib [DAY101] for Treatment of Craniopharyngioma in Children and Young Adults): This study assesses the tolerability and efficacy of combination therapy with PD-1 (nivolumab) and pan-RAF kinase (tovorafenib) inhibition for the treatment of children and young adults with craniopharyngioma.
References:
- Cohen M, Bartels U, Branson H, et al.: Trends in treatment and outcomes of pediatric craniopharyngioma, 1975-2011. Neuro Oncol 15 (6): 767-74, 2013.
- Mortini P, Losa M, Pozzobon G, et al.: Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J Neurosurg 114 (5): 1350-9, 2011.
- Elliott RE, Hsieh K, Hochm T, et al.: Efficacy and safety of radical resection of primary and recurrent craniopharyngiomas in 86 children. J Neurosurg Pediatr 5 (1): 30-48, 2010.
- Zhang YQ, Ma ZY, Wu ZB, et al.: Radical resection of 202 pediatric craniopharyngiomas with special reference to the surgical approaches and hypothalamic protection. Pediatr Neurosurg 44 (6): 435-43, 2008.
- Yang I, Sughrue ME, Rutkowski MJ, et al.: Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus 28 (4): E5, 2010.
- Müller HL, Merchant TE, Puget S, et al.: New outlook on the diagnosis, treatment and follow-up of childhood-onset craniopharyngioma. Nat Rev Endocrinol 13 (5): 299-312, 2017.
- Apps JR, Muller HL, Hankinson TC, et al.: Contemporary Biological Insights and Clinical Management of Craniopharyngioma. Endocr Rev 44 (3): 518-538, 2023.
- Lohkamp LN, Kasper EM, Pousa AE, et al.: An update on multimodal management of craniopharyngioma in children. Front Oncol 13: 1149428, 2023.
- Bogusz A, Müller HL: Childhood-onset craniopharyngioma: latest insights into pathology, diagnostics, treatment, and follow-up. Expert Rev Neurother 18 (10): 793-806, 2018.
- Morisako H, Goto T, Goto H, et al.: Aggressive surgery based on an anatomical subclassification of craniopharyngiomas. Neurosurg Focus 41 (6): E10, 2016.
- Sands SA, Milner JS, Goldberg J, et al.: Quality of life and behavioral follow-up study of pediatric survivors of craniopharyngioma. J Neurosurg 103 (4 Suppl): 302-11, 2005.
- Bakhsheshian J, Jin DL, Chang KE, et al.: Risk factors associated with the surgical management of craniopharyngiomas in pediatric patients: analysis of 1961 patients from a national registry database. Neurosurg Focus 41 (6): E8, 2016.
- Locatelli D, Massimi L, Rigante M, et al.: Endoscopic endonasal transsphenoidal surgery for sellar tumors in children. Int J Pediatr Otorhinolaryngol 74 (11): 1298-302, 2010.
- Chivukula S, Koutourousiou M, Snyderman CH, et al.: Endoscopic endonasal skull base surgery in the pediatric population. J Neurosurg Pediatr 11 (3): 227-41, 2013.
- Mazzatenta D, Zoli M, Guaraldi F, et al.: Outcome of Endoscopic Endonasal Surgery in Pediatric Craniopharyngiomas. World Neurosurg 134: e277-e288, 2020.
- Lee JA, Cooper RL, Nguyen SA, et al.: Endonasal Endoscopic Surgery for Pediatric Sellar and Suprasellar Lesions: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg 163 (2): 284-292, 2020.
- Müller HL, Gebhardt U, Teske C, et al.: Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur J Endocrinol 165 (1): 17-24, 2011.
- Clark AJ, Cage TA, Aranda D, et al.: Treatment-related morbidity and the management of pediatric craniopharyngioma: a systematic review. J Neurosurg Pediatr 10 (4): 293-301, 2012.
- Lin LL, El Naqa I, Leonard JR, et al.: Long-term outcome in children treated for craniopharyngioma with and without radiotherapy. J Neurosurg Pediatr 1 (2): 126-30, 2008.
- Elowe-Gruau E, Beltrand J, Brauner R, et al.: Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab 98 (6): 2376-82, 2013.
- Müller HL: Childhood craniopharyngioma: current controversies on management in diagnostics, treatment and follow-up. Expert Rev Neurother 10 (4): 515-24, 2010.
- Winkfield KM, Tsai HK, Yao X, et al.: Long-term clinical outcomes following treatment of childhood craniopharyngioma. Pediatr Blood Cancer 56 (7): 1120-6, 2011.
- Jimenez RB, Ahmed S, Johnson A, et al.: Proton Radiation Therapy for Pediatric Craniopharyngioma. Int J Radiat Oncol Biol Phys 110 (5): 1480-1487, 2021.
- Eveslage M, Calaminus G, Warmuth-Metz M, et al.: The Postopera tive Quality of Life in Children and Adolescents with Craniopharyngioma. Dtsch Arztebl Int 116 (18): 321-328, 2019.
- Merchant TE, Hua CH, Shukla H, et al.: Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer 51 (1): 110-7, 2008.
- Merchant TE, Kun LE, Hua CH, et al.: Disease control after reduced volume conformal and intensity modulated radiation therapy for childhood craniopharyngioma. Int J Radiat Oncol Biol Phys 85 (4): e187-92, 2013.
- Schoenfeld A, Pekmezci M, Barnes MJ, et al.: The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J Neurooncol 108 (1): 133-9, 2012.
- Edmonston DY, Wu S, Li Y, et al.: Limited surgery and conformal photon radiation therapy for pediatric craniopharyngioma: long-term results from the RT1 protocol. Neuro Oncol 24 (12): 2200-2209, 2022.
- Clark AJ, Cage TA, Aranda D, et al.: A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst 29 (2): 231-8, 2013.
- Beckhaus J, Friedrich C, Boekhoff S, et al.: Outcome after pediatric craniopharyngioma: the role of age at diagnosis and hypothalamic damage. Eur J Endocrinol 188 (3): , 2023.
- Kiehna EN, Merchant TE: Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus 28 (4): E10, 2010.
- Harrabi SB, Adeberg S, Welzel T, et al.: Long term results after fractionated stereotactic radiotherapy (FSRT) in patients with craniopharyngioma: maximal tumor control with minimal side effects. Radiat Oncol 9: 203, 2014.
- Lo AC, Howard AF, Nichol A, et al.: Long-term outcomes and complications in patients with craniopharyngioma: the British Columbia Cancer Agency experience. Int J Radiat Oncol Biol Phys 88 (5): 1011-8, 2014.
- Bishop AJ, Greenfield B, Mahajan A, et al.: Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys 90 (2): 354-61, 2014.
- Winkfield KM, Linsenmeier C, Yock TI, et al.: Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int J Radiat Oncol Biol Phys 73 (3): 716-21, 2009.
- Beltran C, Roca M, Merchant TE: On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int J Radiat Oncol Biol Phys 82 (2): e281-7, 2012.
- Boehling NS, Grosshans DR, Bluett JB, et al.: Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys 82 (2): 643-52, 2012.
- Shi Z, Esiashvili N, Janss AJ, et al.: Transient enlargement of craniopharyngioma after radiation therapy: pattern of magnetic resonance imaging response following radiation. J Neurooncol 109 (2): 349-55, 2012.
- Ishida M, Hotta M, Tsukamura A, et al.: Malignant transformation in craniopharyngioma after radiation therapy: a case report and review of the literature. Clin Neuropathol 29 (1): 2-8, 2010 Jan-Feb.
- Aquilina K, Merchant TE, Rodriguez-Galindo C, et al.: Malignant transformation of irradiated craniopharyngioma in children: report of 2 cases. J Neurosurg Pediatr 5 (2): 155-61, 2010.
- Merchant TE, Hoehn ME, Khan RB, et al.: Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): a single-arm, phase 2 study. Lancet Oncol 24 (5): 523-534, 2023.
- Merchant TE, Dangda S, Hoehn ME, et al.: Pediatric Craniopharyngioma: The Effect of Visual Deficits and Hormone Deficiencies on Long-Term Cognitive Outcomes After Conformal Photon Radiation Therapy. Int J Radiat Oncol Biol Phys 115 (3): 581-591, 2023.
- Bischoff M, Khalil DA, Frisch S, et al.: Outcome After Modern Proton Beam Therapy in Childhood Craniopharyngioma: Results of the Prospective Registry Study KiProReg. Int J Radiat Oncol Biol Phys 120 (1): 137-148, 2024.
- Cinalli G, Spennato P, Cianciulli E, et al.: The role of transventricular neuroendoscopy in the management of craniopharyngiomas: three patient reports and review of the literature. J Pediatr Endocrinol Metab 19 (Suppl 1): 341-54, 2006.
- Schubert T, Trippel M, Tacke U, et al.: Neurosurgical treatment strategies in childhood craniopharyngiomas: is less more? Childs Nerv Syst 25 (11): 1419-27, 2009.
- Julow J, Backlund EO, Lányi F, et al.: Long-term results and late complications after intracavitary yttrium-90 colloid irradiation of recurrent cystic craniopharyngiomas. Neurosurgery 61 (2): 288-95; discussion 295-6, 2007.
- Barriger RB, Chang A, Lo SS, et al.: Phosphorus-32 therapy for cystic craniopharyngiomas. Radiother Oncol 98 (2): 207-12, 2011.
- Maarouf M, El Majdoub F, Fuetsch M, et al.: Stereotactic intracavitary brachytherapy with P-32 for cystic craniopharyngiomas in children. Strahlenther Onkol 192 (3): 157-65, 2016.
- Kickingereder P, Maarouf M, El Majdoub F, et al.: Intracavitary brachytherapy using stereotactically applied phosphorus-32 colloid for treatment of cystic craniopharyngiomas in 53 patients. J Neurooncol 109 (2): 365-74, 2012.
- Ierardi DF, Fernandes MJ, Silva IR, et al.: Apoptosis in alpha interferon (IFN-alpha) intratumoral chemotherapy for cystic craniopharyngiomas. Childs Nerv Syst 23 (9): 1041-6, 2007.
- Cavalheiro S, Di Rocco C, Valenzuela S, et al.: Craniopharyngiomas: intratumoral chemotherapy with interferon-alpha: a multicenter preliminary study with 60 cases. Neurosurg Focus 28 (4): E12, 2010.
- Kilday JP, Caldarelli M, Massimi L, et al.: Intracystic interferon-alpha in pediatric craniopharyngioma patients: an international multicenter assessment on behalf of SIOPE and ISPN. Neuro Oncol 19 (10): 1398-1407, 2017.
- Linnert M, Gehl J: Bleomycin treatment of brain tumors: an evaluation. Anticancer Drugs 20 (3): 157-64, 2009.
- Hukin J, Steinbok P, Lafay-Cousin L, et al.: Intracystic bleomycin therapy for craniopharyngioma in children: the Canadian experience. Cancer 109 (10): 2124-31, 2007.
- Guimarães MM, Cardeal DD, Teixeira MJ, et al.: Brachytherapy in paediatric craniopharyngiomas: a systematic review and meta-analysis of recent literature. Childs Nerv Syst 38 (2): 253-262, 2022.
- Petralia F, Tignor N, Reva B, et al.: Integrated Proteogenomic Characterization across Major Histological Types of Pediatric Brain Cancer. Cell 183 (7): 1962-1985.e31, 2020.
- Apps JR, Carreno G, Gonzalez-Meljem JM, et al.: Tumour compartment transcriptomics demonstrates the activation of inflammatory and odontogenic programmes in human adamantinomatous craniopharyngioma and identifies the MAPK/ERK pathway as a novel therapeutic target. Acta Neuropathol 135 (5): 757-777, 2018.
- Hengartner AC, Prince E, Vijmasi T, et al.: Adamantinomatous craniopharyngioma: moving toward targeted therapies. Neurosurg Focus 48 (1): E7, 2020.
- Coy S, Rashid R, Lin JR, et al.: Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway vulnerabilities in craniopharyngioma. Neuro Oncol 20 (8): 1101-1112, 2018.
- Grob S, Mirsky DM, Donson AM, et al.: Targeting IL-6 Is a Potential Treatment for Primary Cystic Craniopharyngioma. Front Oncol 9: 791, 2019.
- Donson AM, Apps J, Griesinger AM, et al.: Molecular Analyses Reveal Inflammatory Mediators in the Solid Component and Cyst Fluid of Human Adamantinomatous Craniopharyngioma. J Neuropathol Exp Neurol 76 (9): 779-788, 2017.
Treatment of Progressive or Recurrent Childhood Craniopharyngioma
Progression or recurrence of craniopharyngioma varies according to the type of up-front therapy, but it has been reported to be between 20% (patients who received a subtotal resection and radiation therapy) and 90% (patients who received a subtotal resection without radiation therapy).[
Treatment options for recurrent childhood craniopharyngioma include the following:
-
Surgery . -
Radiation therapy, including radiosurgery . -
Intracystic therapy (intracavitary instillation of radioactive phosphorus P 32 [32P] or bleomycin for those with cystic recurrences, where these agents are available) . -
Systemic targeted therapy . -
Observation .
Surgery
The management of recurrent craniopharyngioma is determined largely by previous therapy. Repeat attempts at gross-total resections are difficult, and long-term disease control is achieved less often.[
Radiation Therapy
If not previously employed, external-beam radiation therapy remains an option, including the consideration of radiosurgery in selected circumstances.[
Intracystic Therapy
Cystic recurrences may be treated with intracavitary instillation of varying agents via placement of an Ommaya catheter.[
Systemic and Targeted Therapy
Although systemic therapy is generally not used, a small series has shown that the use of subcutaneous peginterferon alpha-2b to manage cystic recurrences can result in durable responses; however, this agent is no longer commercially available.[
Observation
In select cases of asymptomatic patients with minimal (<25%) tumor progression, it may be possible to safely observe these patients. Intervention can begin when new symptoms develop or further tumor growth is identified on subsequent imaging.[
Treatment Options Under Clinical Evaluation
Information about NCI-supported clinical trials can be found on the
Preclinical contemporary evaluations have identified active molecular and immune pathways in craniopharyngioma that may be targetable using commercially available or investigational agents. Specifically, MAPK and RAF pathways and immune/inflammatory targets such as PD-1 pathway components and IL-6 have been identified.[
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- NCT05465174 (Nivolumab and Tovorafenib [DAY101] for Treatment of Craniopharyngioma in Children and Young Adults): This study assesses the tolerability and efficacy of combination therapy with PD-1 (nivolumab) and pan-RAF kinase (tovorafenib) inhibition for the treatment of children and young adults with craniopharyngioma.
- NCT05286788 (Binimetinib [Mektovi] for the Treatment of Pediatric Adamantinomatous Craniopharyngioma): This phase II study will treat pediatric patients diagnosed with recurrent adamantinomatous craniopharyngioma with binimetinib (mektovi).
- NCT05233397 (Tocilizumab [Actemra] for the Treatment of Progressive or Recurrent Pediatric Adamantinomatous Craniopharyngioma): This phase II study will treat pediatric patients diagnosed with recurrent adamantinomatous craniopharyngioma with tocilizumab (actemra).
References:
- Yang I, Sughrue ME, Rutkowski MJ, et al.: Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus 28 (4): E5, 2010.
- Bogusz A, Müller HL: Childhood-onset craniopharyngioma: latest insights into pathology, diagnostics, treatment, and follow-up. Expert Rev Neurother 18 (10): 793-806, 2018.
- Liubinas SV, Munshey AS, Kaye AH: Management of recurrent craniopharyngioma. J Clin Neurosci 18 (4): 451-7, 2011.
- Vinchon M, Dhellemmes P: Craniopharyngiomas in children: recurrence, reoperation and outcome. Childs Nerv Syst 24 (2): 211-7, 2008.
- Jang WY, Lee KS, Son BC, et al.: Repeat operations in pediatric patients with recurrent craniopharyngiomas. Pediatr Neurosurg 45 (6): 451-5, 2009.
- Xu Z, Yen CP, Schlesinger D, et al.: Outcomes of Gamma Knife surgery for craniopharyngiomas. J Neurooncol 104 (1): 305-13, 2011.
- Foran SJ, Laperriere N, Edelstein K, et al.: Reirradiation for recurrent craniopharyngioma. Adv Radiat Oncol 5 (6): 1305-1310, 2020.
- Kobayashi T: Long-term results of gamma knife radiosurgery for 100 consecutive cases of craniopharyngioma and a treatment strategy. Prog Neurol Surg 22: 63-76, 2009.
- Steinbok P, Hukin J: Intracystic treatments for craniopharyngioma. Neurosurg Focus 28 (4): E13, 2010.
- Julow J, Backlund EO, Lányi F, et al.: Long-term results and late complications after intracavitary yttrium-90 colloid irradiation of recurrent cystic craniopharyngiomas. Neurosurgery 61 (2): 288-95; discussion 295-6, 2007.
- Barriger RB, Chang A, Lo SS, et al.: Phosphorus-32 therapy for cystic craniopharyngiomas. Radiother Oncol 98 (2): 207-12, 2011.
- Maarouf M, El Majdoub F, Fuetsch M, et al.: Stereotactic intracavitary brachytherapy with P-32 for cystic craniopharyngiomas in children. Strahlenther Onkol 192 (3): 157-65, 2016.
- Kickingereder P, Maarouf M, El Majdoub F, et al.: Intracavitary brachytherapy using stereotactically applied phosphorus-32 colloid for treatment of cystic craniopharyngiomas in 53 patients. J Neurooncol 109 (2): 365-74, 2012.
- Linnert M, Gehl J: Bleomycin treatment of brain tumors: an evaluation. Anticancer Drugs 20 (3): 157-64, 2009.
- Hukin J, Steinbok P, Lafay-Cousin L, et al.: Intracystic bleomycin therapy for craniopharyngioma in children: the Canadian experience. Cancer 109 (10): 2124-31, 2007.
- Ierardi DF, Fernandes MJ, Silva IR, et al.: Apoptosis in alpha interferon (IFN-alpha) intratumoral chemotherapy for cystic craniopharyngiomas. Childs Nerv Syst 23 (9): 1041-6, 2007.
- Cavalheiro S, Di Rocco C, Valenzuela S, et al.: Craniopharyngiomas: intratumoral chemotherapy with interferon-alpha: a multicenter preliminary study with 60 cases. Neurosurg Focus 28 (4): E12, 2010.
- Kilday JP, Caldarelli M, Massimi L, et al.: Intracystic interferon-alpha in pediatric craniopharyngioma patients: an international multicenter assessment on behalf of SIOPE and ISPN. Neuro Oncol 19 (10): 1398-1407, 2017.
- Yeung JT, Pollack IF, Panigrahy A, et al.: Pegylated interferon-α-2b for children with recurrent craniopharyngioma. J Neurosurg Pediatr 10 (6): 498-503, 2012.
- Fouda MA, Karsten M, Staffa SJ, et al.: Management strategies for recurrent pediatric craniopharyngioma: new recommendations. J Neurosurg Pediatr 27 (5): 548-555, 2021.
- Petralia F, Tignor N, Reva B, et al.: Integrated Proteogenomic Characterization across Major Histological Types of Pediatric Brain Cancer. Cell 183 (7): 1962-1985.e31, 2020.
- Apps JR, Muller HL, Hankinson TC, et al.: Contemporary Biological Insights and Clinical Management of Craniopharyngioma. Endocr Rev 44 (3): 518-538, 2023.
- Apps JR, Carreno G, Gonzalez-Meljem JM, et al.: Tumour compartment transcriptomics demonstrates the activation of inflammatory and odontogenic programmes in human adamantinomatous craniopharyngioma and identifies the MAPK/ERK pathway as a novel therapeutic target. Acta Neuropathol 135 (5): 757-777, 2018.
- Hengartner AC, Prince E, Vijmasi T, et al.: Adamantinomatous craniopharyngioma: moving toward targeted therapies. Neurosurg Focus 48 (1): E7, 2020.
- Coy S, Rashid R, Lin JR, et al.: Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway vulnerabilities in craniopharyngioma. Neuro Oncol 20 (8): 1101-1112, 2018.
- Grob S, Mirsky DM, Donson AM, et al.: Targeting IL-6 Is a Potential Treatment for Primary Cystic Craniopharyngioma. Front Oncol 9: 791, 2019.
- Donson AM, Apps J, Griesinger AM, et al.: Molecular Analyses Reveal Inflammatory Mediators in the Solid Component and Cyst Fluid of Human Adamantinomatous Craniopharyngioma. J Neuropathol Exp Neurol 76 (9): 779-788, 2017.
Late Effects in Patients Treated for Childhood Craniopharyngioma
Quality-of-life issues are important to pediatric patients with craniopharyngiomas and are difficult to generalize because of the various treatment modalities. In one series of 261 patients diagnosed with craniopharyngiomas before 2000, hypothalamic involvement was associated with lower overall survival (OS), impaired quality of life, and severe obesity.[
Late effects of treatment for childhood craniopharyngioma include the following:
- Behavioral issues and memory deficits. Although intelligence quotient is usually maintained, behavioral issues and other cognitive domains such as memory, executive function, and attention are commonly impacted.[
4 ,6 ,7 ,8 ] Memory and neurocognitive effects may be mitigated by the use of proton radiation therapy and conformal plans to avoid surrounding normal brain anatomy such as the hypothalamus and hippocampus.[9 ]; [10 ][Level of evidence B3] - Visual disturbances. Visual disturbances, including visual field and acuity defects, have been reported. These deficits may be decreased with less aggressive surgical approaches or radiation therapy alone.[
11 ][Level of evidence C1]; [6 ,10 ] - Endocrine abnormalities. Endocrine abnormalities result in the almost universal need for lifelong endocrine replacement with multiple pituitary hormones.[
5 ,8 ]; [12 ,13 ,14 ][Level of evidence C1] Similar to visual disturbances, endocrine injury can be offset by limited surgical resection [5 ,6 ,15 ,16 ,17 ] and intracystic therapies that minimize invasive interventions.[18 ] - Decreased height. Growth hormone replacement therapy is used to improve growth in children treated for craniopharyngiomas. Growth hormone replacement initiated in childhood results in increases in height without impact on OS and progression-free survival when compared with children who did not receive growth hormone.[
19 ][Level of evidence C1]; [20 ] Growth hormone administration beginning 1 year after diagnosis may be associated with early improvements in quality of life when measured at 3 years postdiagnosis.[21 ][Level of evidence C1] Published consensus guidelines do not support an increased risk of recurrence with use of growth hormones. They recommend considering growth hormone replacement therapy as early as 3 months after completing cancer therapy in patients who have stable disease and significant growth deficits.[22 ][Level of evidence D] - Obesity. Obesity, which can be life-threatening, and the development of metabolic syndrome, including nonalcoholic fatty liver disease, can occur.[
23 ,24 ] Children who undergo complete resection or subtotal resection may develop obesity, suggesting that a predilection to obesity may be a component of the disease itself, not the result of direct hypothalamic injury.[25 ][Level of evidence C1] Severe obesity seen in patients with craniopharyngiomas is more likely a result of a combination of factors such as tumor location and treatment characteristics, with multifaceted downstream impacts.[26 ][Level of evidence C1] In a study of 709 patients with craniopharyngiomas, posterior hypothalamic involvement or operative injury to the posterior hypothalamus appeared to be a key factor in the development of severe obesity.[3 ] - Vasculopathies and stroke. Vasculopathies and subsequent strokes may result from local irradiation.[
27 ,28 ] Previous studies have suggested that long-term growth hormone replacement may reduce the risk of stroke. Studies have also shown that pretreatment characteristics such as existing vascular injury, vessel location in the surgical field, and larger radiation doses to vascular structures increase the risk of long-term vessel stenosis.[28 ]; [29 ][Level of evidence C1] In a study of 94 pediatric patients with craniopharyngiomas who were treated with surgery and 54 Gy of proton therapy, the strongest predictor of postradiation therapy vasculopathy was preexisting vasculopathy.[29 ] The impact of proton radiation therapy was negligible within the operative corridor. Despite the high incidence (n = 27, 28.7%) of imaging-only evidence of subclinical stenosis events, only five patients required a revascularization procedure. In one of these patients, high-grade stenosis was present before radiation therapy. Two patients had previous tumor recurrences that required multiple resections before radiation therapy. - Subsequent neoplasms. Subsequent neoplasms may result from local irradiation.[
27 ] Secondary malignancies related to radiation therapy that specifically involve the pituitary/sellar region can range from malignant tumors, such as high-grade gliomas, to meningiomas. This risk is increased in patients who are younger at the time of radiation therapy.[30 ]
For information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors, see
References:
- Sterkenburg AS, Hoffmann A, Gebhardt U, et al.: Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: newly reported long-term outcomes. Neuro Oncol 17 (7): 1029-38, 2015.
- Eveslage M, Calaminus G, Warmuth-Metz M, et al.: The Postopera tive Quality of Life in Children and Adolescents with Craniopharyngioma. Dtsch Arztebl Int 116 (18): 321-328, 2019.
- Beckhaus J, Friedrich C, Boekhoff S, et al.: Outcome after pediatric craniopharyngioma: the role of age at diagnosis and hypothalamic damage. Eur J Endocrinol 188 (3): , 2023.
- Apps JR, Muller HL, Hankinson TC, et al.: Contemporary Biological Insights and Clinical Management of Craniopharyngioma. Endocr Rev 44 (3): 518-538, 2023.
- Müller HL: Childhood craniopharyngioma: current controversies on management in diagnostics, treatment and follow-up. Expert Rev Neurother 10 (4): 515-24, 2010.
- Bogusz A, Müller HL: Childhood-onset craniopharyngioma: latest insights into pathology, diagnostics, treatment, and follow-up. Expert Rev Neurother 18 (10): 793-806, 2018.
- Winkfield KM, Tsai HK, Yao X, et al.: Long-term clinical outcomes following treatment of childhood craniopharyngioma. Pediatr Blood Cancer 56 (7): 1120-6, 2011.
- Giese H, Haenig B, Haenig A, et al.: Neurological and neuropsychological outcome after resection of craniopharyngiomas. J Neurosurg 132 (5): 1425-1434, 2019.
- Özyurt J, Thiel CM, Lorenzen A, et al.: Neuropsychological outcome in patients with childhood craniopharyngioma and hypothalamic involvement. J Pediatr 164 (4): 876-881.e4, 2014.
- Merchant TE, Hoehn ME, Khan RB, et al.: Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): a single-arm, phase 2 study. Lancet Oncol 24 (5): 523-534, 2023.
- Wan MJ, Zapotocky M, Bouffet E, et al.: Long-term visual outcomes of craniopharyngioma in children. J Neurooncol 137 (3): 645-651, 2018.
- Vinchon M, Weill J, Delestret I, et al.: Craniopharyngioma and hypothalamic obesity in children. Childs Nerv Syst 25 (3): 347-52, 2009.
- Dolson EP, Conklin HM, Li C, et al.: Predicting behavioral problems in craniopharyngioma survivors after conformal radiation therapy. Pediatr Blood Cancer 52 (7): 860-4, 2009.
- Kawamata T, Amano K, Aihara Y, et al.: Optimal treatment strategy for craniopharyngiomas based on long-term functional outcomes of recent and past treatment modalities. Neurosurg Rev 33 (1): 71-81, 2010.
- Müller HL: Consequences of craniopharyngioma surgery in children. J Clin Endocrinol Metab 96 (7): 1981-91, 2011.
- Marcus HJ, Rasul FT, Hussein Z, et al.: Craniopharyngioma in children: trends from a third consecutive single-center cohort study. J Neurosurg Pediatr 25 (3): 242-250, 2019.
- Clark AJ, Cage TA, Aranda D, et al.: Treatment-related morbidity and the management of pediatric craniopharyngioma: a systematic review. J Neurosurg Pediatr 10 (4): 293-301, 2012.
- Lohkamp LN, Kasper EM, Pousa AE, et al.: An update on multimodal management of craniopharyngioma in children. Front Oncol 13: 1149428, 2023.
- Boekhoff S, Bogusz A, Sterkenburg AS, et al.: Long-term Effects of Growth Hormone Replacement Therapy in Childhood-onset Craniopharyngioma: Results of the German Craniopharyngioma Registry (HIT-Endo). Eur J Endocrinol 179 (5): 331-341, 2018.
- Nguyen Quoc A, Beccaria K, González Briceño L, et al.: GH and Childhood-onset Craniopharyngioma: When to Initiate GH Replacement Therapy? J Clin Endocrinol Metab 108 (8): 1929-1936, 2023.
- Heinks K, Boekhoff S, Hoffmann A, et al.: Quality of life and growth after childhood craniopharyngioma: results of the multinational trial KRANIOPHARYNGEOM 2007. Endocrine 59 (2): 364-372, 2018.
- Boguszewski MCS, Boguszewski CL, Chemaitilly W, et al.: Safety of growth hormone replacement in survivors of cancer and intracranial and pituitary tumours: a consensus statement. Eur J Endocrinol 186 (6): P35-P52, 2022.
- Elowe-Gruau E, Beltrand J, Brauner R, et al.: Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab 98 (6): 2376-82, 2013.
- Hoffmann A, Bootsveld K, Gebhardt U, et al.: Nonalcoholic fatty liver disease and fatigue in long-term survivors of childhood-onset craniopharyngioma. Eur J Endocrinol 173 (3): 389-97, 2015.
- Tan TS, Patel L, Gopal-Kothandapani JS, et al.: The neuroendocrine sequelae of paediatric craniopharyngioma: a 40-year meta-data analysis of 185 cases from three UK centres. Eur J Endocrinol 176 (3): 359-369, 2017.
- Otte A, Müller HL: Childhood-onset Craniopharyngioma. J Clin Endocrinol Metab 106 (10): e3820-e3836, 2021.
- Kiehna EN, Merchant TE: Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus 28 (4): E10, 2010.
- Lo AC, Howard AF, Nichol A, et al.: A Cross-Sectional Cohort Study of Cerebrovascular Disease and Late Effects After Radiation Therapy for Craniopharyngioma. Pediatr Blood Cancer 63 (5): 786-93, 2016.
- Lucas JT, Faught AM, Hsu CY, et al.: Pre- and Posttherapy Risk Factors for Vasculopathy in Pediatric Patients With Craniopharyngioma Treated With Surgery and Proton Radiation Therapy. Int J Radiat Oncol Biol Phys 113 (1): 152-160, 2022.
- Burman P, van Beek AP, Biller BM, et al.: Radiotherapy, Especially at Young Age, Increases the Risk for De Novo Brain Tumors in Patients Treated for Pituitary/Sellar Lesions. J Clin Endocrinol Metab 102 (3): 1051-1058, 2017.
Latest Updates to This Summary (11 / 26 / 2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Added
Added Nguyen Quoc et al. as
This summary is written and maintained by the
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood craniopharyngioma. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Craniopharyngioma Treatment are:
- Kenneth J. Cohen, MD, MBA (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital)
- Karen J. Marcus, MD, FACR (Dana-Farber of Boston Children's Cancer Center and Blood Disorders Harvard Medical School)
- Roger J. Packer, MD (Children's National Hospital)
- D. Williams Parsons, MD, PhD (Texas Children's Hospital)
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Craniopharyngioma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at:
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our
Last Revised: 2024-11-26
This information does not replace the advice of a doctor. Ignite Healthwise, LLC, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.
Page Footer
I want to...
Audiences
Secure Member Sites
The Cigna Group Information
Disclaimer
Individual and family medical and dental insurance plans are insured by Cigna Health and Life Insurance Company (CHLIC), Cigna HealthCare of Arizona, Inc., Cigna HealthCare of Illinois, Inc., Cigna HealthCare of Georgia, Inc., Cigna HealthCare of North Carolina, Inc., Cigna HealthCare of South Carolina, Inc., and Cigna HealthCare of Texas, Inc. Group health insurance and health benefit plans are insured or administered by CHLIC, Connecticut General Life Insurance Company (CGLIC), or their affiliates (see
All insurance policies and group benefit plans contain exclusions and limitations. For availability, costs and complete details of coverage, contact a licensed agent or Cigna sales representative. This website is not intended for residents of New Mexico.