Shop for Plans
Shop for your own coverage
Plans through your employer
Learn about the medical, dental, pharmacy, behavioral, and voluntary benefits your employer may offer.
Learn
Living or working abroad?
Childhood Liver Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]
General Information About Childhood Liver Cancer
Liver cancer is a rare malignancy in children and adolescents and is divided into the following two major histological subgroups:
-
Hepatoblastoma .Well-differentiated fetal (pure fetal) histology .- Mixed epithelial and fetal histology.
Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver .- Small cell undifferentiated hepatoblastoma (SMARCB1 positive).
- Rhabdoid tumor of liver (SMARCB1 negative).
-
Hepatocellular carcinoma .
Other less common histologies include the following:
-
Fibrolamellar carcinoma . -
Undifferentiated embryonal sarcoma of the liver . -
Infantile choriocarcinoma of the liver . -
Vascular liver tumors . - Biliary rhabdomyosarcoma. For more information, see
Childhood Rhabdomyosarcoma Treatment .
Harmonization of Childhood Liver Cancer Data and Definitions
Historically, four major study groups have performed prospective clinical trials in children with liver tumors: The International Childhood Liver Tumors Strategy Group (previously known as Société Internationale d'Oncologie Pédiatrique–Epithelial Liver Tumor Study Group [SIOPEL]), the Gesellschaft für Pädiatrische Onkologie und Hämatologie (Society for Paediatric Oncology and Haematology [GPOH]), the Japanese Study Group for Pediatric Liver Tumors (JPLT), and the Children's Oncology Group (COG), including its predecessor groups the Children's Cancer Group (CCG) and Pediatric Oncology Group (POG). These groups historically had disparate risk stratification categories, data elements that were monitored, and pathological and radiological definitions, making it difficult to compare outcomes across continents.
A collaborative effort among all four study groups collated their disparate data into a unified database called the Children's Hepatic Tumor International Collaboration (CHIC). The CHIC group analyzed clinical features and outcomes in a database that included 1,605 patients with hepatoblastoma treated in eight separate multicenter clinical trials, with central review of all tumor imaging and histological details.[
References:
- Czauderna P, Haeberle B, Hiyama E, et al.: The Children's Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer 52: 92-101, 2016.
- Otte JB, Pritchard J, Aronson DC, et al.: Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer 42 (1): 74-83, 2004.
Cellular Classification of Childhood Liver Cancer
Liver tumors are rare in children. A definitive pathological diagnosis may be challenging because of the rarity of the tumor and the lack of a universal classification system before the Children's Hepatic Tumor International Collaboration (CHIC) harmonization efforts. Systematic central histopathological review of these tumors, performed as part of pediatric collaborative therapeutic protocols, has allowed the identification of histological subtypes with distinct clinical associations.
The Children's Oncology Group (COG) Liver Tumor Committee sponsored an International Pathology Symposium in 2011 to discuss the histopathology and classification of pediatric liver tumors (hepatoblastoma, in particular) and develop an International Pediatric Liver Tumors Consensus Classification that would be required for international collaborative projects. The results were published in 2014.[
For information about the histology of each childhood liver cancer subtype, see the following sections:
-
Hepatoblastoma . -
Hepatocellular carcinoma . -
Fibrolamellar carcinoma . -
Undifferentiated embryonal sarcoma of the liver . -
Infantile choriocarcinoma of the liver . -
Vascular liver tumors .
References:
- López-Terrada D, Alaggio R, de Dávila MT, et al.: Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 27 (3): 472-91, 2014.
- Cho SJ, Ranganathan S, Alaggio R, et al.: Consensus classification of pediatric hepatocellular tumors: A report from the Children's Hepatic tumors International Collaboration (CHIC). Pediatr Blood Cancer : e30505, 2023.
Tumor Stratification by Imaging
A main treatment goal for children and adolescents with liver cancer is surgical extirpation of the primary tumor. Risk grouping depends heavily on factors determined by imaging that are related to safe surgical resection of the tumor, as well as the PRETEXT grouping. These imaging findings include the section or sections of the liver that are involved with the tumor and additional findings, called annotation factors, that impact surgical decision making and prognosis.
Risk stratification of children and adolescents with liver cancer involves the use of high-quality, cross-sectional imaging. Three-phase computed tomography scanning (noncontrast, arterial, and venous) or magnetic resonance imaging (MRI) with contrast agents are used. MRI with gadoxetate disodium, a gadolinium-based agent that is preferentially taken up and excreted by hepatocytes, is being used with increased frequency and may improve detection of multifocal disease.[
PRETEXT and POSTTEXT Group Definitions
The imaging grouping systems used to radiologically define the extent of liver involvement by the tumor are designated as the following:
- PRETEXT (PRE-Treatment EXTent of disease): The extent of liver involvement is defined before therapy.
- POSTTEXT (POST-Treatment EXTent of disease): The extent of liver involvement is defined in response to therapy.
PRETEXT
Major multicenter trial groups use PRETEXT as a central component of risk stratification schemes that guide treatment of hepatoblastoma. PRETEXT is based on the Couinaud eight-segment anatomical structure of the liver using cross-sectional imaging.
The PRETEXT system divides the liver into four parts, called sections. The left lobe of the liver consists of a lateral section (Couinaud segments I, II, and III) and a medial section (segment IV), whereas the right lobe consists of an anterior section (segments V and VIII) and a posterior section (segments VI and VII) (see
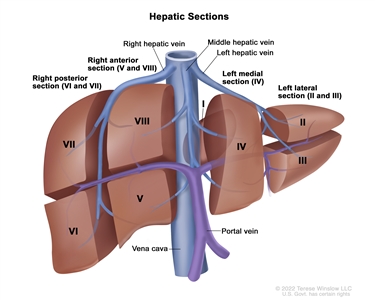
Figure 1. The liver is divided into four sections: the right posterior section, the right anterior section, the left medial section, and the left lateral section. Each section of the liver is further divided into segments: segments VI and VII make up the right posterior section, segments V and VIII make up the right anterior section, segment IV makes up the left medial section, and segments II and III make up the left lateral section. Segment I is found deep in the left side of the liver, in front of the inferior vena cava and behind the right, middle, and left hepatic veins.
PRETEXT group assignment I, II, III, or IV is determined by the number of uninvolved sections of the liver. PRETEXT is further described by annotation factors. Annotation factors include findings that are important for surgical management and evidence of tumor extension beyond the hepatic parenchyma of the major sections, including metastatic disease. For detailed descriptions of the PRETEXT groups, see
Annotation factors identify the extent of tumor involvement of the major vessels and its effect on venous inflow and outflow. These factors provide critical knowledge for the surgeon and can affect surgical outcomes. At one time, definitions of gross vascular involvement used by the Children's Oncology Group (COG) and major liver surgery centers in the United States differed from those used by SIOPEL and in Europe. These differences have been resolved, and the new definitions are being used in an international trial.[
Although PRETEXT can be used to predict tumor resectability, it has limitations. It can be difficult to distinguish real invasion beyond the anatomical border of a given hepatic section from compression and displacement by the tumor, especially at diagnosis. Additionally, it can be difficult to distinguish between vessel encroachment and involvement, particularly if imaging is inadequate. The PRETEXT group assignment has a moderate degree of interobserver variability. In a report using data from the SIOPEL-1 study, the preoperative PRETEXT group aligned with postoperative pathological findings only 51% of the time, with overstaging in 37% of patients and understaging in 12% of patients.[
Because distinguishing PRETEXT group assignment is difficult, central review of imaging is critical and is generally performed in all major clinical trials. For patients not enrolled in clinical trials, expert radiological review should be considered in questionable cases in which the PRETEXT group assignment affects choice of treatment.
| PRETEXT and POSTTEXT Groups | Definition | Image | |
|---|---|---|---|
| a Adapted from Roebuck et al.[ |
|||
| I | One section involved; three adjoining sections are tumor free. | 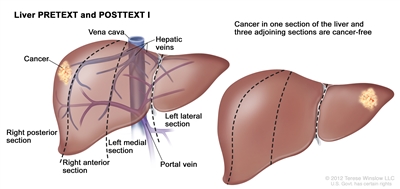 |
|
| II | One or two sections involved; two adjoining sections are tumor free. | 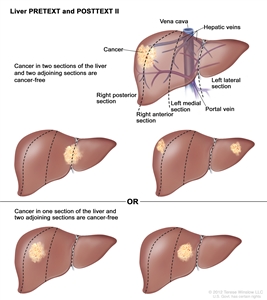 |
|
| III | Two or three sections involved; one adjoining section is tumor free. | 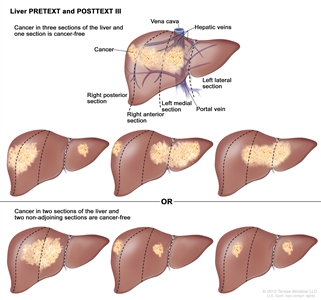 |
|
| IV | Four sections involved. | 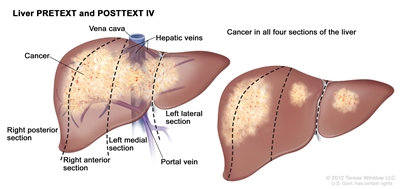 |
|
| Annotation Factors | Definition | ||
|---|---|---|---|
| CT = computed tomography; MRI = magnetic resonance imaging; HU = Hounsfield unit. | |||
| a Adapted from Roebuck et al.[ |
|||
| b Additional details describing the annotation factors have been published.[ |
|||
| Vb | Venous involvement: Vascular involvement of the retrohepatic vena cava or involvement ofall three major hepatic veins (right, middle, and left). | ||
| V0 | Tumor within 1 cm. | ||
| V1 | Tumor abutting. | ||
| V2 | Tumor compressing or distorting. | ||
| V3 | Tumor ingrowth, encasement, or thrombus. | ||
| Pb | Portal involvement: Vascular involvement of the main portal vein and/orboth right and left portal veins. | ||
| P0 | Tumor within 1 cm. | ||
| P1 | Tumor abutting the main portal vein, the right and left portal veins, or the portal vein bifurcation. | ||
| P2 | Tumor compressing the main portal vein, the right and left portal veins, or the portal vein bifurcation. | ||
| P3 | Tumor ingrowth, encasement (>50% or >180 degrees), or intravascular thrombus within the main portal vein, the right and left portal veins, or the portal vein bifurcation. | ||
| Eb | Extrahepatic spread of disease. Any one of the following criteria is met: | ||
| E1 | Tumor crosses boundaries/tissue planes. | ||
| E2 | Tumor is surrounded by normal tissue more than 180 degrees. | ||
| E3 | Peritoneal nodules (not lymph nodes) are present so that there is at least one nodule measuring ≥10 mm or at least two nodules measuring ≥5 mm. | ||
| Mb | Distant metastases. Any one of the following criteria is met: | ||
| M1 | One noncalcified pulmonary nodule ≥5 mm in diameter. | ||
| M2 | Two or more noncalcified pulmonary nodules, each ≥3 mm in diameter. | ||
| M3 | Pathologically proven metastatic disease. | ||
| C | Tumor involving the caudate. | ||
| F | Multifocality. Two or more discrete hepatic tumors with normal intervening liver tissue. | ||
| Nb | Lymph node metastases. Any one of the following criteria is met: | ||
| N1 | Lymph node with short-axis diameter of >1 cm. | ||
| N2 | Portocaval lymph node with short-axis diameter >1.5 cm. | ||
| N3 | Spherical lymph node shape with loss of fatty hilum. | ||
| Rb | Tumor rupture. Free fluid in the abdomen or pelvis with one or more of the following findings of hemorrhage: | ||
| R1 | Internal complexity/septations within fluid. | ||
| R2 | High-density fluid on CT (>25 HU). | ||
| R3 | Imaging characteristics of blood or blood degradation products on MRI. | ||
| R4 | Heterogeneous fluid on ultrasound with echogenic debris. | ||
| R5 | Visible defect in tumor capsuleOR tumor cells are present within the peritoneal fluidOR rupture diagnosed pathologically in patients who have received an upfront resection. | ||
POSTTEXT
The POSTTEXT group is determined after patients receive chemotherapy. The greatest chemotherapy response, measured as decreases in tumor size and alpha-fetoprotein (AFP) level, occurs after the first two cycles of chemotherapy.[
Evans Surgical Staging for Childhood Liver Cancer
The COG/Evans staging system, based on operative findings and surgical resectability, was used for many years in the United States to group and determine treatment for children with liver cancer (see
| Evans Surgical Stage | Definition |
|---|---|
| Stage I | The tumor is completely resected. |
| Stage II | Microscopic residual tumor remains after resection. |
| Stage III | There are no distant metastases and at least one of the following is true: (1) the tumor is either unresectable or the tumor is resected with gross residual tumor; (2) there are positive extrahepatic lymph nodes. |
| Stage IV | There is distant metastasis, regardless of the extent of liver involvement. |
References:
- Meyers AB, Towbin AJ, Geller JI, et al.: Hepatoblastoma imaging with gadoxetate disodium-enhanced MRI--typical, atypical, pre- and post-treatment evaluation. Pediatr Radiol 42 (7): 859-66, 2012.
- Brown J, Perilongo G, Shafford E, et al.: Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 36 (11): 1418-25, 2000.
- Roebuck DJ, Aronson D, Clapuyt P, et al.: 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol 37 (2): 123-32; quiz 249-50, 2007.
- Towbin AJ, Meyers RL, Woodley H, et al.: 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol 48 (4): 536-554, 2018.
- Aronson DC, Schnater JM, Staalman CR, et al.: Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol 23 (6): 1245-52, 2005.
- Lovvorn HN, Ayers D, Zhao Z, et al.: Defining hepatoblastoma responsiveness to induction therapy as measured by tumor volume and serum alpha-fetoprotein kinetics. J Pediatr Surg 45 (1): 121-8; discussion 129, 2010.
- Venkatramani R, Stein JE, Sapra A, et al.: Effect of neoadjuvant chemotherapy on resectability of stage III and IV hepatoblastoma. Br J Surg 102 (1): 108-13, 2015.
- Ortega JA, Krailo MD, Haas JE, et al.: Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: a report from the Childrens Cancer Study Group. J Clin Oncol 9 (12): 2167-76, 1991.
- Douglass EC, Reynolds M, Finegold M, et al.: Cisplatin, vincristine, and fluorouracil therapy for hepatoblastoma: a Pediatric Oncology Group study. J Clin Oncol 11 (1): 96-9, 1993.
- Ortega JA, Douglass EC, Feusner JH, et al.: Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol 18 (14): 2665-75, 2000.
Treatment Option Overview for Childhood Liver Cancer
Many of the improvements in survival in childhood cancer have been made using new therapies that have attempted to improve on the best available, accepted therapy. Clinical trials in pediatrics are designed to compare potentially better therapy with therapy that is currently accepted as standard. This comparison may be done in a randomized study of two treatment arms or by evaluating a single new treatment and comparing the results with those previously obtained with standard therapy.
Because of the relative rarity of cancer in children, all children with liver cancer should be considered for a clinical trial if available. Treatment planning by a multidisciplinary team of cancer specialists with experience treating tumors of childhood is required to determine and implement optimal treatment.[
Surgery
Historically, complete surgical resection of the primary tumor has been essential for cure of malignant liver tumors in children.[
The three surgical options to treat primary pediatric liver cancer include the following:
- Initial surgical resection (alone or with adjuvant chemotherapy).
- Delayed surgical resection (with neoadjuvant chemotherapy).
- Orthotopic liver (cadaveric and living donor) transplant (most often with neoadjuvant chemotherapy).
The decision on which surgical approach to use (e.g., partial hepatectomy, extended resection, or transplant) depends on many factors, including the following:
- PRE-Treatment EXTent of disease (PRETEXT) group and POST-Treatment EXTent of disease (POSTTEXT) group.
- Size of the primary tumor.
- Presence of multifocal hepatic disease.
- Gross vascular involvement.
- Alpha-fetoprotein (AFP) levels.
- Whether preoperative chemotherapy is likely to convert an unresectable tumor into a resectable tumor.
- Whether hepatic disease meets surgical and histopathological criteria for orthotopic liver transplant.
Timing of the surgical approach is critical. Surgeons who have experience performing pediatric liver resections and transplants are involved early in the decision-making process to determine optimal timing and extent of resection.
Early involvement, preferably at diagnosis, with an experienced pediatric liver surgeon is especially important in patients with PRETEXT group III or IV or involvement of major liver vessels (positive annotation factors V [venous] or P [portal]).[
Intraoperative ultrasonography may result in further delineation of tumor extent and location and can affect intraoperative management.[
If the tumor is determined to be unresectable, measures to reduce the tumor size to make a complete surgical resection possible need to be considered. These measures include preoperative intravenous chemotherapy, transarterial chemotherapy, or transarterial radioactive therapy. These efforts must be carefully coordinated with the surgical team to facilitate planning of resection. Prolonged chemotherapy can lead to unnecessary delays and, in rare cases, tumor progression. If the tumor can be completely excised by an experienced surgical team, less postoperative chemotherapy may be needed. Incomplete resection must be avoided because patients who undergo rescue transplants of incompletely resected tumors have an inferior outcome, compared with patients who undergo transplant as the primary surgical therapy.[
The approach taken by the Children's Oncology Group (COG) in North American clinical trials is to perform surgery initially when a complete resection can be done with a simple, negative-margin hemihepatectomy. The COG AHEP0731 (NCT00980460) trial studied the use of PRETEXT and POSTTEXT to determine the optimal approach and timing of surgery. POSTTEXT imaging grouping was performed after two and four cycles of chemotherapy to determine the optimal time for definitive surgery.[
Orthotopic liver transplant
Liver transplants have been associated with significant success in the treatment of children with unresectable hepatic tumors.[
Evidence (orthotopic liver transplant):
- The United Network for Organ Sharing (UNOS) database was queried for all patients younger than 18 years with a primary malignant liver tumor who underwent an orthotopic liver transplant between 1987 and 2012 (N = 544). The patients were diagnosed with hepatoblastoma (n = 376, 70%), hepatocellular carcinoma (n = 84, 15%), and other tumors (n = 84, 15%). Patients with hepatocellular carcinoma were older, more often hospitalized at the time of transplant, and more likely to receive a cadaveric organ than were patients with hepatoblastoma.[
27 ]- The 5-year patient survival rate was 73%, and the graft survival rate was 74% for the entire cohort, with most deaths resulting from malignancy. On multivariate analysis, independent predictors of 5-year patient and graft survival included the following:
- Diagnosis.
- For the study period of 1987 to 2012, the 5-year survival rate was 76% and the graft survival rate was 77% for patients with hepatoblastoma. The survival and graft survival rates were 63% for patients with hepatocellular carcinoma.
- For the study period of 2009 to 2012, the 3-year survival and graft survival rates were 84% for patients with hepatoblastoma. The survival and graft survival rates were 85% for patients with hepatocellular carcinoma.
- Transplant era.
- The death rate by hazard ratio was 1.0 for the period before 2002, 0.72 for the period of 2002 to 2009, and 0.54 for the period of 2009 to 2012.
- Medical condition at transplant.
- For hepatoblastoma patients, the survival rate by hazard ratio was 1.0 for hospitalized patients versus 1.81 for nonhospitalized patients at the time of transplant.
- For hepatocellular carcinoma patients, the survival rate by hazard ratio was 1.0 for hospitalized patients versus 1.92 for nonhospitalized patients.
- Patients hospitalized in the intensive care unit did not fare worse than patients not in the intensive care unit.
- Diagnosis.
- The 5-year patient survival rate was 73%, and the graft survival rate was 74% for the entire cohort, with most deaths resulting from malignancy. On multivariate analysis, independent predictors of 5-year patient and graft survival included the following:
- A report of 149 patients with hepatocellular carcinoma younger than 21 years who underwent transplants between 1987 and 2015 used detailed data collected at all U.S. pediatric transplant centers.[
19 ]- The 1-year graft survival rate of about 85% did not differ from the survival rate for patients with hepatoblastoma or biliary atresia. Survival rates continued to decline over time, from 85% at 1 year to 52% at 5 years and 43% at 10 years, a more dramatic decline than that seen for hepatoblastoma or biliary atresia.
- The survival after transplant did not differ from that of adults who underwent transplant for hepatocellular carcinoma.
- Of the patients with hepatocellular carcinoma, 22 received a diagnosis after transplant for medical cirrhotic disease such as tyrosinemia. They had a superior outcome, but it was not statistically significant compared with the rest of the patients.
- A review of the Surveillance, Epidemiology, and End Results (SEER) Program database and many single-institution series have reported results similar to the UNOS database study described above.[
11 ,20 ,21 ,22 ,28 ]; [25 ][Level of evidence C1] - In a three-institution study of children with hepatocellular carcinoma, the overall 5-year disease-free survival rate was approximately 60%.[
29 ] - In a study that used the Society of Pediatric Liver Transplantation (SPLIT) database to identify patients who underwent liver transplant between 2011 and 2019, the following was reported:[
30 ][Level of evidence C2]- The 3-year event-free survival (EFS) rate was 81% for patients with hepatoblastoma who received a transplant (n = 157).
- The 3-year EFS rate was 62% for patients with hepatocellular carcinoma who received a transplant (n = 18).
- Of the patients who received a transplant to treat hepatoblastoma, 6.9% had PRETEXT II disease and 15.3% had POSTTEXT I/II disease.
- Tumor extent did not impact survival (P = NS).
- Patients who received transplants for salvage (n = 13) and patients who received transplants for primary hepatoblastoma had similar 3-year EFS rates (62% vs. 78%; P = NS).
- Among patients who received transplants for hepatocellular carcinoma, the 3-year EFS rate was poorer in older patients (38% for patients aged ≥8 years vs. 86% for patients aged <8 years; P < .001).
Application of the Milan criteria for UNOS selection of recipients of deceased donor livers is controversial.[
Cirrhosis is an underlying risk factor for the development of hepatocellular carcinoma in children who suffer from certain diseases or conditions. These diseases include perinatally acquired hepatitis B, hepatorenal tyrosinemia, progressive familial intrahepatic cholestasis, glycogen storage disease, Alagille syndrome, and other conditions. Improvements in screening methodology have allowed for earlier identification and treatment of some of these conditions, as well as monitoring for development of hepatocellular carcinoma. Nevertheless, because of the poor prognosis of patients with hepatocellular carcinoma, liver transplant should be considered for diseases or conditions that have resulted in early findings of cirrhosis, before the development of liver failure or malignancy.[
Living-donor liver transplant for hepatic malignancy is more common in children than adults, and the outcome is similar to those undergoing cadaveric liver transplant.[
Surgical resection for metastatic disease
Surgical resection of metastatic disease is often recommended, but the rate of cure in children with hepatoblastoma has not been fully determined. Resection of metastases may be done for areas of locally invasive disease (e.g., diaphragm) and isolated brain metastases. Resection of pulmonary metastases should be considered if the number of metastases is limited.[
Radiofrequency ablation has also been used to treat oligometastatic hepatoblastoma when patients prefer to avoid surgical metastasectomy.[
Chemotherapy
Chemotherapy regimens used in the treatment of hepatoblastoma and hepatocellular carcinoma are described in their respective sections. Chemotherapy has been much more successful in the treatment of hepatoblastoma than in the treatment of hepatocellular carcinoma.[
The standard of care in the United States is preoperative chemotherapy when the tumor is unresectable and postoperative chemotherapy after complete resection, even if preoperative chemotherapy has already been given.[
Radiation Therapy
Radiation therapy, even in combination with chemotherapy, has not cured children with unresectable hepatic tumors. A study of 154 patients with hepatoblastoma showed that radiation therapy and/or second resection of positive margins may not be necessary in some patients with incompletely resected hepatoblastoma and microscopic residual tumor.[
Other Treatment Approaches
Other treatment approaches include the following:
- Transarterial chemoembolization (TACE): TACE is an image-guided, minimally invasive, nonsurgical procedure that is used to treat malignant lesions in the liver. The procedure uses a catheter to deliver both chemotherapy medication and embolization materials into the blood vessels that lead to the tumor. The arterial catheter route is image guided, most often via the hepatic artery, and perfusion of the tumor by the targeted artery may be confirmed by imaging before therapeutic injection. This procedure allows for the treatment of tumors that are not accessible with conventional surgery or radiation treatments. TACE has been used for patients with inoperable hepatoblastoma.[
47 ,48 ,49 ] This procedure has also been used in a few children to successfully shrink tumors to permit resections.[48 ] - Transarterial radioembolization (TARE): TARE is an image-guided, minimally invasive, nonsurgical procedure that delivers radiation therapy to treat tumors in the liver. This procedure delivers radioactive beads and blocks arterial flow within the tumor to keep the radiation inside the tumor. Glass or resin microspheres, coated most commonly with yttrium Y 90 (90Y), are delivered to the tumor via catheters placed in arteries that supply the tumor. Usually, the hepatic artery or its branches are used, but tumors may be partially supplied by parasitized surrounding vessels. Because of the risk of radiation delivery to the nearby lung, technetium Tc 99m microaggregated albumin imaging is performed with delivery via the catheter that is in place before the administration of radioactive beads to carefully measure radiation exposure to the lung. If calculations determine that lung exposure is unsafe, TARE is not pursued. TARE with 90Y has been used in children with hepatoblastoma (n = 2) and hepatocellular carcinoma (n = 2) who have unresectable tumors. After treatment with 90Y TARE, all tumors were completely resected.[
50 ][Level of evidence C3]; [51 ][Level of evidence C2] This approach has also been used for palliation in children with hepatocellular carcinoma.[52 ] For more information, seePrimary Liver Cancer Treatment . - High-intensity focused ultrasonography (HIFU): HIFU is a noninvasive treatment for a wide range of tumors and diseases. HIFU uses an ultrasound transducer, similar to the ones used for diagnostic imaging, but with much higher energy. The transducer focuses sound waves to generate heat at a single point in the body and destroy the target tissue. The tissue can get as hot as 66°C in only 20 seconds. This process is repeated as many times as necessary until the target tissue is destroyed. Magnetic resonance imaging is used to plan the treatment and monitor the amount of heat in real time. A combination of chemotherapy followed by TACE and HIFU showed promising results in China for children with PRETEXT III and PRETEXT IV malignant liver tumors, some of whom had resectable tumors but did not undergo surgery because of parent refusal.[
53 ]
References:
- Tiao GM, Bobey N, Allen S, et al.: The current management of hepatoblastoma: a combination of chemotherapy, conventional resection, and liver transplantation. J Pediatr 146 (2): 204-11, 2005.
- Czauderna P, Otte JB, Aronson DC, et al.: Guidelines for surgical treatment of hepatoblastoma in the modern era--recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL). Eur J Cancer 41 (7): 1031-6, 2005.
- Czauderna P, Mackinlay G, Perilongo G, et al.: Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol 20 (12): 2798-804, 2002.
- Meyers RL, Czauderna P, Otte JB: Surgical treatment of hepatoblastoma. Pediatr Blood Cancer 59 (5): 800-8, 2012.
- Aronson DC, Meyers RL: Malignant tumors of the liver in children. Semin Pediatr Surg 25 (5): 265-275, 2016.
- Murawski M, Weeda VB, Maibach R, et al.: Hepatocellular Carcinoma in Children: Does Modified Platinum- and Doxorubicin-Based Chemotherapy Increase Tumor Resectability and Change Outcome? Lessons Learned From the SIOPEL 2 and 3 Studies. J Clin Oncol 34 (10): 1050-6, 2016.
- Allan BJ, Wang B, Davis JS, et al.: A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg 49 (1): 166-71; discussion 171, 2014.
- Becker K, Furch C, Schmid I, et al.: Impact of postoperative complications on overall survival of patients with hepatoblastoma. Pediatr Blood Cancer 62 (1): 24-8, 2015.
- D'Antiga L, Vallortigara F, Cillo U, et al.: Features predicting unresectability in hepatoblastoma. Cancer 110 (5): 1050-8, 2007.
- Lautz TB, Ben-Ami T, Tantemsapya N, et al.: Successful nontransplant resection of POST-TEXT III and IV hepatoblastoma. Cancer 117 (9): 1976-83, 2011.
- Fonseca A, Gupta A, Shaikh F, et al.: Extreme hepatic resections for the treatment of advanced hepatoblastoma: Are planned close margins an acceptable approach? Pediatr Blood Cancer 65 (2): , 2018.
- Fuchs J, Cavdar S, Blumenstock G, et al.: POST-TEXT III and IV Hepatoblastoma: Extended Hepatic Resection Avoids Liver Transplantation in Selected Cases. Ann Surg 266 (2): 318-323, 2017.
- Baertschiger RM, Ozsahin H, Rougemont AL, et al.: Cure of multifocal panhepatic hepatoblastoma: is liver transplantation always necessary? J Pediatr Surg 45 (5): 1030-6, 2010.
- Felsted AE, Shi Y, Masand PM, et al.: Intraoperative ultrasound for liver tumor resection in children. J Surg Res 198 (2): 418-23, 2015.
- Rossi G, Tarasconi A, Baiocchi G, et al.: Fluorescence guided surgery in liver tumors: applications and advantages. Acta Biomed 89 (9-S): 135-140, 2018.
- Shen Q, Liu X, Pan S, et al.: Effectiveness of indocyanine green fluorescence imaging in resection of hepatoblastoma. Pediatr Surg Int 39 (1): 181, 2023.
- Otte JB, Pritchard J, Aronson DC, et al.: Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer 42 (1): 74-83, 2004.
- Venkatramani R, Stein JE, Sapra A, et al.: Effect of neoadjuvant chemotherapy on resectability of stage III and IV hepatoblastoma. Br J Surg 102 (1): 108-13, 2015.
- Vinayak R, Cruz RJ, Ranganathan S, et al.: Pediatric liver transplantation for hepatocellular cancer and rare liver malignancies: US multicenter and single-center experience (1981-2015). Liver Transpl 23 (12): 1577-1588, 2017.
- Guiteau JJ, Cotton RT, Karpen SJ, et al.: Pediatric liver transplantation for primary malignant liver tumors with a focus on hepatic epithelioid hemangioendothelioma: the UNOS experience. Pediatr Transplant 14 (3): 326-31, 2010.
- Malek MM, Shah SR, Atri P, et al.: Review of outcomes of primary liver cancers in children: our institutional experience with resection and transplantation. Surgery 148 (4): 778-82; discussion 782-4, 2010.
- Héry G, Franchi-Abella S, Habes D, et al.: Initial liver transplantation for unresectable hepatoblastoma after chemotherapy. Pediatr Blood Cancer 57 (7): 1270-5, 2011.
- Suh MY, Wang K, Gutweiler JR, et al.: Safety of minimal immunosuppression in liver transplantation for hepatoblastoma. J Pediatr Surg 43 (6): 1148-52, 2008.
- Zsíros J, Maibach R, Shafford E, et al.: Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol 28 (15): 2584-90, 2010.
- Khan AS, Brecklin B, Vachharajani N, et al.: Liver Transplantation for Malignant Primary Pediatric Hepatic Tumors. J Am Coll Surg 225 (1): 103-113, 2017.
- Browne M, Sher D, Grant D, et al.: Survival after liver transplantation for hepatoblastoma: a 2-center experience. J Pediatr Surg 43 (11): 1973-81, 2008.
- Hamilton EC, Balogh J, Nguyen DT, et al.: Liver transplantation for primary hepatic malignancies of childhood: The UNOS experience. J Pediatr Surg : , 2017.
- McAteer JP, Goldin AB, Healey PJ, et al.: Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatr Transplant 17 (8): 744-50, 2013.
- Reyes JD, Carr B, Dvorchik I, et al.: Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr 136 (6): 795-804, 2000.
- Boster JM, Superina R, Mazariegos GV, et al.: Predictors of survival following liver transplantation for pediatric hepatoblastoma and hepatocellular carcinoma: Experience from the Society of Pediatric Liver Transplantation (SPLIT). Am J Transplant 22 (5): 1396-1408, 2022.
- Otte JB: Should the selection of children with hepatocellular carcinoma be based on Milan criteria? Pediatr Transplant 12 (1): 1-3, 2008.
- de Ville de Goyet J, Meyers RL, Tiao GM, et al.: Beyond the Milan criteria for liver transplantation in children with hepatic tumours. Lancet Gastroenterol Hepatol 2 (6): 456-462, 2017.
- Khanna R, Verma SK: Pediatric hepatocellular carcinoma. World J Gastroenterol 24 (35): 3980-3999, 2018.
- Sevmis S, Karakayali H, Ozçay F, et al.: Liver transplantation for hepatocellular carcinoma in children. Pediatr Transplant 12 (1): 52-6, 2008.
- Faraj W, Dar F, Marangoni G, et al.: Liver transplantation for hepatoblastoma. Liver Transpl 14 (11): 1614-9, 2008.
- Pire A, Tambucci R, De Magnée C, et al.: Living donor liver transplantation for hepatic malignancies in children. Pediatr Transplant 25 (7): e14047, 2021.
- Feusner JH, Krailo MD, Haas JE, et al.: Treatment of pulmonary metastases of initial stage I hepatoblastoma in childhood. Report from the Childrens Cancer Group. Cancer 71 (3): 859-64, 1993.
- Zsiros J, Brugieres L, Brock P, et al.: Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol 14 (9): 834-42, 2013.
- Meyers RL, Katzenstein HM, Krailo M, et al.: Surgical resection of pulmonary metastatic lesions in children with hepatoblastoma. J Pediatr Surg 42 (12): 2050-6, 2007.
- O'Neill AF, Towbin AJ, Krailo MD, et al.: Characterization of Pulmonary Metastases in Children With Hepatoblastoma Treated on Children's Oncology Group Protocol AHEP0731 (The Treatment of Children With All Stages of Hepatoblastoma): A Report From the Children's Oncology Group. J Clin Oncol 35 (30): 3465-3473, 2017.
- Yevich S, Calandri M, Gravel G, et al.: Reiterative Radiofrequency Ablation in the Management of Pediatric Patients with Hepatoblastoma Metastases to the Lung, Liver, or Bone. Cardiovasc Intervent Radiol 42 (1): 41-47, 2019.
- Weeda VB, Murawski M, McCabe AJ, et al.: Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma--results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer 49 (12): 2698-704, 2013.
- Czauderna P, Lopez-Terrada D, Hiyama E, et al.: Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr 26 (1): 19-28, 2014.
- Schnater JM, Aronson DC, Plaschkes J, et al.: Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer 94 (4): 1111-20, 2002.
- Habrand JL, Nehme D, Kalifa C, et al.: Is there a place for radiation therapy in the management of hepatoblastomas and hepatocellular carcinomas in children? Int J Radiat Oncol Biol Phys 23 (3): 525-31, 1992.
- Wang PM, Chung NN, Hsu WC, et al.: Stereotactic body radiation therapy in hepatocellular carcinoma: Optimal treatment strategies based on liver segmentation and functional hepatic reserve. Rep Pract Oncol Radiother 20 (6): 417-24, 2015 Nov-Dec.
- Xianliang H, Jianhong L, Xuewu J, et al.: Cure of hepatoblastoma with transcatheter arterial chemoembolization. J Pediatr Hematol Oncol 26 (1): 60-3, 2004.
- Malogolowkin MH, Stanley P, Steele DA, et al.: Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol 18 (6): 1279-84, 2000.
- Hirakawa M, Nishie A, Asayama Y, et al.: Efficacy of preoperative transcatheter arterial chemoembolization combined with systemic chemotherapy for treatment of unresectable hepatoblastoma in children. Jpn J Radiol 32 (9): 529-36, 2014.
- Aguado A, Dunn SP, Averill LW, et al.: Successful use of transarterial radioembolization with yttrium-90 (TARE-Y90) in two children with hepatoblastoma. Pediatr Blood Cancer 67 (9): e28421, 2020.
- Whitlock RS, Loo C, Patel K, et al.: Transarterial Radioembolization Treatment as a Bridge to Surgical Resection in Pediatric Hepatocellular Carcinoma. J Pediatr Hematol Oncol 43 (8): e1181-e1185, 2021.
- Hawkins CM, Kukreja K, Geller JI, et al.: Radioembolisation for treatment of pediatric hepatocellular carcinoma. Pediatr Radiol 43 (7): 876-81, 2013.
- Wang S, Yang C, Zhang J, et al.: First experience of high-intensity focused ultrasound combined with transcatheter arterial embolization as local control for hepatoblastoma. Hepatology 59 (1): 170-7, 2014.
Special Considerations for the Treatment of Children With Cancer
Cancer in children and adolescents is rare, although the overall incidence has slowly increased since 1975.[
- Primary care physicians.
- Pediatric surgeons.
- Transplant surgeons.
- Pathologists.
- Pediatric radiation oncologists.
- Pediatric medical oncologists and hematologists.
- Ophthalmologists.
- Rehabilitation specialists.
- Pediatric oncology nurses.
- Social workers.
- Child-life professionals.
- Psychologists.
- Nutritionists.
For specific information about supportive care for children and adolescents with cancer, see the summaries on
The American Academy of Pediatrics has outlined guidelines for pediatric cancer centers and their role in the treatment of children and adolescents with cancer.[
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2020, childhood cancer mortality decreased by more than 50%.[
References:
- Smith MA, Seibel NL, Altekruse SF, et al.: Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28 (15): 2625-34, 2010.
- American Academy of Pediatrics: Standards for pediatric cancer centers. Pediatrics 134 (2): 410-4, 2014.
Also available online . Last accessed February 25, 2025. - Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.
- National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute.
Available online . Last accessed February 25, 2025. - Surveillance Research Program, National Cancer Institute: SEER*Explorer: An interactive website for SEER cancer statistics. Bethesda, MD: National Cancer Institute.
Available online . Last accessed December 30, 2024.
Hepatoblastoma
Incidence
The annual incidence of hepatoblastoma in the United States has increased (more than doubled), from 0.8 (1975–1983) to 2.3 (2020) cases per 1 million children aged 19 years and younger.[
The age of onset of liver cancer in children is related to tumor histology. Hepatoblastomas usually occur before the age of 3 years, and approximately 90% of malignant liver tumors in children aged 4 years and younger are hepatoblastomas.[
Risk Factors
Conditions associated with an increased risk of hepatoblastoma are described in
| Associated Disorder | Clinical Findings |
|---|---|
| Aicardi syndrome[ |
For more information, see the |
| Beckwith-Wiedemann syndrome[ |
For more information, see the |
| Familial adenomatous polyposis[ |
For more information, see the |
| Glycogen storage diseases I–IV[ |
Symptoms vary by individual disorder. |
| Low-birth-weight infants[ |
Preterm and small-for-gestation-age neonates. |
| Simpson-Golabi-Behmel syndrome[ |
Macroglossia, macrosomia, renal and skeletal abnormalities, and increased risk of Wilms tumor. |
| Trisomy 18, other trisomies[ |
Trisomy 18: Microcephaly and micrognathia, clenched fists with overlapping fingers, and failure to thrive. Most patients (>90%) die in the first year of life. |
Aicardi syndrome
Aicardi syndrome is presumed to be an X-linked condition reported exclusively in females, leading to the hypothesis that an altered gene on the X chromosome causes lethality in males. The syndrome is classically defined as agenesis of the corpus callosum, chorioretinal lacunae, and infantile spasms, with a characteristic facies. Additional brain, eye, and costovertebral defects are often found.[
Beckwith-Wiedemann syndrome and hemihyperplasia
The incidence of hepatoblastoma increases 1,000-fold to 10,000-fold in infants and children with Beckwith-Wiedemann syndrome.[
Beckwith-Wiedemann syndrome is most commonly caused by epigenetic changes and is sporadic. The syndrome may also be caused by genetic variants and be familial. Either mechanism can be associated with an increased incidence of embryonal tumors, including Wilms tumor and hepatoblastoma.[
To detect abdominal malignancies at an early stage, all children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia undergo regular screening for multiple tumor types by abdominal ultrasonography.[
Familial adenomatous polyposis
Hepatoblastoma is associated with familial adenomatous polyposis (FAP). Children in families that carry the APC gene have an 800-fold increased risk of hepatoblastoma. Screening for hepatoblastoma in members of families with FAP using ultrasonography and AFP levels is controversial because hepatoblastoma has been reported to occur in less than 1% of this group.[
Current evidence cannot rule out the possibility that predisposition to hepatoblastoma may be limited to a specific subset of APC variants. Another study of children with hepatoblastoma found a predominance of the variant in the 5' region of the gene, but some patients had variants closer to the 3' region.[
In the absence of APC germline variants, childhood hepatoblastomas do not have somatic variants in the APC gene. However, hepatoblastomas frequently have variants in the CTNNB1 gene, whose function is closely related to APC.[
Screening children predisposed to hepatoblastoma
An American Association for Cancer Research publication suggested that all children with genetic syndromes that lead to a risk of 1% or greater for developing hepatoblastoma undergo screening. This group includes patients with Beckwith-Wiedemann syndrome, hemihyperplasia, Simpson-Golabi-Behmel syndrome, and trisomy 18 syndrome. Screening is by abdominal ultrasonography and AFP determination every 3 months from birth (or diagnosis) through the fourth birthday, which will identify 90% to 95% of hepatoblastomas that develop in these children.[
Genomics of Hepatoblastoma
Molecular features of hepatoblastoma
Genomic findings related to hepatoblastoma include the following:
- The frequency of variants in hepatoblastoma, as determined by three groups using whole-exome sequencing, was very low (approximately three variants per tumor) in children younger than 5 years.[
31 ,32 ,33 ,34 ] A pediatric pan-cancer genomics study found that hepatoblastoma had the lowest gene variant rate among all childhood cancers studied.[35 ] - Hepatoblastoma is primarily a disease of WNT pathway activation. The primary mechanism for WNT pathway activation is CTNNB1 activating variants/deletions involving exon 3. CTNNB1 variants have been reported in more than 80% of cases.[
31 ,33 ,34 ,36 ,37 ] A less common cause of WNT pathway activation in hepatoblastoma is variants in APC associated with familial adenomatosis polyposis coli.[36 ] - NFE2L2 variants were identified in 10 of 174 (6%), 4 of 88 (5%), and 5 of 112 (4%) cases of hepatoblastoma in three studies.[
33 ,34 ,37 ] The presence of NFE2L2 variants was associated with a lower survival rate.[37 ] - Similar NFE2L2 variants have been found in many types of cancer, including hepatocellular carcinoma. These variants render NFE2L2 insensitive to KEAP1-mediated degradation, leading to activation of the NFE2L2-KEAP1 pathway, which activates resistance to oxidative stress and is believed to confer resistance to chemotherapy.
- TERT and TP53 variants, which are common in adults with hepatocellular carcinoma,[
38 ] are uncommon in children with hepatoblastoma.[31 ,33 ,34 ,36 ] Pediatric patients with TERT variants present with hepatoblastoma at a significantly older age, compared with patients without TERT variants (median age at diagnosis, approximately 10 years vs. 1.4 years).[37 ] - Uniparental disomy at 11p15.5 with loss of the maternal allele was reported in 6 of 15 cases of hepatoblastoma.[
39 ] This finding has been confirmed in genomic characterization studies, in which 30% to 40% of cases showed allelic imbalance at the 11p15 locus.[34 ,36 ,37 ]
Gene expression and epigenetic profiling have been used to identify biological subtypes of hepatoblastoma and to evaluate the prognostic significance of these subtypes.[
- A 16-gene expression signature divided hepatoblastoma cases into two subsets,[
37 ,40 ] C1 and C2. The C1 subtype included most of the well-differentiated fetal (pure fetal) histology cases. The C2 subtype showed a more immature pattern and was associated with higher rates of metastatic disease at diagnosis. In a study of 174 patients with hepatoblastoma, the C2 subtype was a significant predictor of poor outcome in multivariable analysis.[37 ] - A second research group also found that gene expression profiling could be used to identify subsets of hepatoblastoma with favorable versus unfavorable prognosis.[
33 ] The unfavorable prognosis group of patients showed elevated expression of genes associated with embryonic stem cell and progenitor cells (e.g., LIN28B, SALL4, and HMGA2). The favorable prognosis group of patients showed elevated expression of genes associated with liver differentiation (e.g., HNF1A). - A gene expression signature at chromosome 14q32 (e.g., DLK1) was identified, with a stronger expression signal being associated with higher risk of treatment failure.[
34 ] A strong 14q32 expression signature was also observed in fetal liver tissue, further supporting the concept that patients with hepatoblastoma who have tumors with biological characteristics that are similar to those of hepatic precursor cells have an inferior prognosis. - Epigenetic profiling of hepatoblastoma has been used to identify molecularly defined hepatoblastoma subtypes. Tumors from 113 patients with hepatoblastoma were evaluated using DNA methylation arrays. Two distinctive subtypes were identified, epigenetic cluster A and B (Epi-CA and Epi-CB).[
34 ] The methylation profile of Epi-CB resembled that of early embryonal/fetal phases of liver development. The methylation profile of Epi-CA was similar to that of late fetal or postnatal liver phases. Event-free survival was significantly lower for patients with the Epi-CB subtype than for those with the Epi-CA subtype.[34 ]
Delineating the clinical applications of these genomic, transcriptomic, and epigenomic profiling methods for the risk classification of patients with hepatoblastoma will require independent validation, which is one of the objectives of the Paediatric Hepatic International Tumour Trial (PHITT [NCT03017326]).
Diagnosis
Biopsy
A biopsy is always indicated to confirm the diagnosis of a pediatric liver tumor, except in the following circumstances:
- Infantile hepatic hemangioma. Biopsy is not indicated for patients with infantile hemangioma of the liver with classic findings on magnetic resonance imaging (MRI). If the diagnosis is in doubt after high-quality imaging, a confirmatory biopsy is done.
- Focal nodular hyperplasia. Biopsy may not be indicated or may be delayed for patients with focal nodular hyperplasia with classic features on MRI using hepatocyte-specific contrast agent. If the diagnosis is in doubt, a confirmatory biopsy is done.
- Children's Oncology Group (COG) surgical guidelines (AHEP0731 [NCT00980460] appendix) recommend tumor resection at diagnosis without preoperative chemotherapy in children with PRE-Treatment EXTent of disease (PRETEXT) group I tumors and PRETEXT group II tumors with greater than 1 cm radiographic margin on the vena cava and middle hepatic and portal veins. Therefore, biopsy is not usually recommended in this circumstance.
- Infantile hepatic choriocarcinoma. In patients with infantile hepatic choriocarcinoma, which can be diagnosed by imaging and markedly elevated beta-human chorionic gonadotropin (beta-hCG), chemotherapy without biopsy is often indicated.[
41 ]
Tumor markers
The AFP and beta-hCG tumor markers are helpful in the diagnosis and management of liver tumors. Although AFP is elevated in most children with hepatic malignancies, it is not pathognomonic for a malignant liver tumor.[
Prognosis and Prognostic Factors
Prognosis
The 5-year overall survival (OS) rate for children with hepatoblastoma is 70%.[
Survival rates at 5 years, unrelated to annotation factors, were found to be the following:
- 90% for patients with PRETEXT I group tumors.
- 83% for patients with PRETEXT II group tumors.
- 73% for patients with PRETEXT III group tumors.
- 52% for patients with PRETEXT IV group tumors.
When each annotation factor was examined separately, regardless of the PRETEXT group or other annotation factors, the 5-year OS rates were found to be the following:
- 51% for patients with positive V (involvement of all three hepatic veins and/or inferior vena cava).
- 49% for patients with positive P (involvement of both right and left portal veins).
- 53% for patients with positive E (contiguous extrahepatic tumor).
- 52% for patients with positive F (multifocal).
- 51% for patients with positive R (tumor rupture).
- 41% for patients with positive M (distant metastasis).
For more information about PRETEXT grouping and annotation factors, see the
Hepatoblastoma prognosis by Evans surgical stage. Current study protocols use the PRETEXT staging for prognosis. The prognosis, based on Evans stage, is listed below. For more information, see the
- Stages I and II.
Approximately 20% to 30% of children with hepatoblastoma have stage I or II disease. Prognosis varies depending on the subtype of hepatoblastoma:
- Patients with well-differentiated fetal (previously termed pure fetal) histology tumors (4% of hepatoblastomas) have a 3- to 5-year OS rate of 100% with minimal or no chemotherapy, whether PRETEXT I, II, or III.[
50 ,51 ,52 ] - Patients with non–well-differentiated fetal histology, non–small cell undifferentiated stage I and II hepatoblastomas have a 3- to 4-year OS rate of 90% to 100% with adjuvant chemotherapy.[
50 ,51 ] - If any small cell undifferentiated elements are present in patients with stage I or II hepatoblastoma, the 3-year survival rate is 40% to 70%.[
50 ,53 ]
- Patients with well-differentiated fetal (previously termed pure fetal) histology tumors (4% of hepatoblastomas) have a 3- to 5-year OS rate of 100% with minimal or no chemotherapy, whether PRETEXT I, II, or III.[
- Stage III.
Approximately 50% to 70% of children with hepatoblastoma have stage III disease. The 3- to 5-year OS rate for these children is less than 70%.[
50 ,51 ] - Stage IV.
Approximately 10% to 20% of children with hepatoblastoma have stage IV disease. The 3- to 5-year OS rate for these children varies widely, from 20% to approximately 60%, based on published reports.[
50 ,51 ,54 ,55 ,56 ,57 ] Postsurgical stage IV is equivalent to any PRETEXT group with annotation factor M.[58 ,59 ,60 ]
Prognostic factors
Individual childhood cancer study groups have attempted to define the relative importance of a variety of prognostic factors present at diagnosis and in response to therapy.[
- Higher PRETEXT group.[
58 ] - Positive PRETEXT annotation factors:[
58 ]- V: Involvement of all three hepatic veins and/or intrahepatic inferior vena cava.
- P: Involvement of both left and right portal veins.
- E: Contiguous extrahepatic tumor extensions (e.g., diaphragm, adjacent organs).
- F: Multifocal tumors.
- R: Tumor rupture.
- M: Distant metastases, usually lung.
- Low AFP level (<100 ng/mL or 100–1,000 ng/mL to account for infants with elevated AFP levels).[
63 ] - Older age. Patients aged 3 to 7 years have a worse outcome in the PRETEXT IV group.[
58 ] Patients aged 8 years and older have a worse outcome than younger patients in all PRETEXT groups. In a subsequent report from the CHIC group, risk of an event increased with advancing age throughout all age cohorts.[64 ][Level of evidence C1] Increasing age attenuated the effect of other risk factors, including metastasis, AFP level less than 100 ng/mL, tumor rupture, and the presence of one annotation factor.In contrast, in the SIOPEL-2 and -3 studies, infants younger than 6 months had PRETEXT group, annotation factors, and outcomes similar to those of older children undergoing the same treatment.[
65 ][Level of evidence C1]
In the CHIC study, sex, prematurity, birth weight, and Beckwith-Wiedemann syndrome had no effect on EFS.[
A multivariate analysis of these prognostic factors was published to help develop a new risk group classification for hepatoblastoma.[
Other studies observed the following factors that affected prognosis:
- PRETEXT group: In SIOPEL studies, having a low PRETEXT group at diagnosis (PRETEXT I, II, and III tumors) is a good prognostic factor, whereas PRETEXT IV is a poor prognostic factor.[
58 ] For more information, see theTumor Stratification by Imaging section. - Tumor stage: In COG studies, patients with classical hepatoblastoma histology and stage I tumors that were resected at diagnosis have a favorable outcome when treated with limited chemotherapy. Patients with tumors that have well-differentiated fetal histology have an excellent prognosis. These tumors are not generally treated with chemotherapy. Patients with tumors of other stages and histologies are treated more aggressively.[
58 ] - Treatment-related factors:
Chemotherapy: Chemotherapy often decreases the size and extent of hepatoblastoma tumors, allowing complete resection.[
51 ,54 ,66 ,67 ,68 ] Favorable response of the primary tumor to chemotherapy predicts its resectability, with favorable response defined as either a 30% decrease in tumor size by Response Evaluation Criteria In Solid Tumors (RECIST) or 90% or greater decrease in AFP levels. In turn, this favorable response predicted OS among all CHIC risk groups treated with neoadjuvant chemotherapy in the JPLT-2 Japanese national clinical trial.[69 ][Level of evidence B4]Surgery: Cure of hepatoblastoma requires gross tumor resection. Hepatoblastoma is most often unifocal, so resection may be possible. Most patients survive if a hepatoblastoma is completely removed. However, because of vascular or other involvement, less than one-third of patients have lesions that are amenable to complete resection at diagnosis.[
58 ] It is critically important that a child with probable hepatoblastoma be evaluated by a pediatric surgeon who is experienced in the techniques of extreme liver resection with vascular reconstruction. The child should also have access to a liver transplant program. In advanced tumors, surgical treatment of hepatoblastoma is a demanding procedure. Postoperative complications in high-risk patients decrease the OS rate.[70 ]Orthotopic liver transplant: Orthotopic liver transplant is an additional treatment option for patients whose tumor remains unresectable after preoperative chemotherapy.[
71 ,72 ] However, the presence of microscopic residual tumor at the surgical margin does not preclude a favorable outcome.[73 ,74 ] This outcome may result from additional courses of chemotherapy administered before or after resection.[66 ,67 ,73 ]For more information about the outcomes associated with specific chemotherapy regimens, see
Table 6 . - Tumor marker–related factors:
Ninety percent of children with hepatoblastoma and two-thirds of children with hepatocellular carcinoma exhibit elevated levels of the serum tumor marker AFP, which parallels disease activity. The level of AFP at diagnosis and rate of decrease in AFP levels during treatment are compared with the age-adjusted reference range. Lack of a significant decrease in AFP levels with treatment may predict a poor response to therapy.[
75 ] In an exploratory study of 34 children with hepatoblastoma, the rate of decrease in AFP and tumor volume, but not in RECIST I measurements, following two courses of treatment after diagnosis was predictive of EFS and OS.[76 ]Absence of elevated AFP levels at diagnosis (AFP <100 ng/mL) occurs in a small percentage of children with hepatoblastoma and appears to be associated with very poor prognosis, as well as with the small cell undifferentiated variant of hepatoblastoma.[
58 ] Some of these variants do not express SMARCB1 and may be considered rhabdoid tumors of the liver, which require alternative therapy. All small cell undifferentiated hepatoblastomas are tested for loss of SMARCB1 expression by immunohistochemistry to determine those that should be treated as a hepatoblastoma versus those that should be treated as rhabdoid tumors of the liver.[50 ,53 ,56 ,57 ,77 ,78 ]Beta-hCG levels may also be elevated in children with hepatoblastoma or hepatocellular carcinoma, which may result in isosexual precocity in boys.[
45 ,46 ] - Tumor histology:
For more information, see the
Histology section in the Hepatoblastoma section.
Other variables have been proposed to be poor prognostic factors, but their significance has been difficult to define. In the SIOPEL-1 study, a multivariate analysis of prognosis after positive response to chemotherapy showed that only one variable, PRETEXT group, predicted OS, while metastasis and PRETEXT group predicted EFS.[
Histology
Hepatoblastoma arises from precursors of hepatocytes and can have several morphologies, including the following:[
- Small cells that reflect neither epithelial nor stromal differentiation. It is critical to discriminate between small cell undifferentiated hepatoblastoma expressing SMARCB1 and rhabdoid tumor of the liver, which lacks the SMARCB1 gene and SMARCB1 expression. Both diseases may share similar histology. Optimal treatment of rhabdoid tumor of the liver and small cell undifferentiated hepatoblastoma may require different approaches and different chemotherapy. For a more extensive discussion on the differences of these two diseases, see the
Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver section. - Embryonal epithelial cells resembling the liver epithelium at 6 to 8 weeks of gestation.
- Well-differentiated fetal hepatocytes morphologically indistinguishable from normal fetal liver cells.
Most often the tumor consists of a mixture of epithelial hepatocyte precursors. About 20% of tumors have stromal derivatives such as osteoid, chondroid, and rhabdoid elements. Occasionally, neuronal, melanocytic, squamous, and enteroendocrine elements are found. The following histological subtypes have clinical relevance:
-
Well-differentiated fetal (pure fetal) histology hepatoblastoma . - Mixed fetal and embryonal epithelial cells.
-
Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver .- Small cell undifferentiated hepatoblastoma (SMARCB1 positive).
- Rhabdoid tumor of liver (SMARCB1 negative).
Well-differentiated fetal (pure fetal) histology hepatoblastoma
An analysis of patients with initially resected hepatoblastoma tumors (before receiving chemotherapy) has suggested that patients with well-differentiated fetal (previously termed pure fetal) histology tumors have a better prognosis than patients with an admixture of more primitive and rapidly dividing embryonal components or other undifferentiated tissues. Studies have reported the following:
- A study of patients with hepatoblastoma and well-differentiated fetal histology tumors observed the following:[
51 ]- The survival rate was 100% for patients who received four doses of single-agent doxorubicin. This finding suggested that patients with well-differentiated fetal histology tumors might not need chemotherapy after complete resection.[
81 ,82 ]
- The survival rate was 100% for patients who received four doses of single-agent doxorubicin. This finding suggested that patients with well-differentiated fetal histology tumors might not need chemotherapy after complete resection.[
- In a COG study (COG-P9645), 16 patients with well-differentiated fetal histology hepatoblastoma with two or fewer mitoses per 10 high-power fields were not treated with chemotherapy. Retrospectively, their PRETEXT groups were group I (n = 4), group II (n = 6), and group III (n = 2).[
52 ]- The survival rate was 100%.
- All 16 patients were alive with no evidence of disease at a median follow-up of 4.9 years (range, 9 months to 9.2 years).
Thus, complete resection of a well-differentiated fetal hepatoblastoma may preclude the need for chemotherapy.
Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver
Small cell undifferentiated hepatoblastoma (SMARCB1 retained) is an uncommon hepatoblastoma variant. Histologically, small cell undifferentiated hepatoblastoma is typified by a diffuse population of small cells with scant cytoplasm resembling neuroblasts.[
Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the livers can be distinguished by the following characteristic abnormalities:
- Chromosomal abnormalities. These abnormalities in rhabdoid tumors include translocations involving a breakpoint on chromosome 22q11 and homozygous deletion at the chromosome 22q12 region that harbors the SMARCB1 gene.[
84 ,85 ] - Lack of SMARCB1 expression. Lack of detection of SMARCB1 by immunohistochemistry is characteristic of malignant rhabdoid tumors.[
84 ]
Historically, small cell undifferentiated hepatoblastoma was reported to occur at a younger age (6–10 months) than other cases of hepatoblastoma [
The Paediatric Hepatic International Tumour Trial (PHITT) designates any childhood liver tumor as rhabdoid tumor of the liver if it contains cells that lack SMARCB1 expression. Patients with SMARCB1-negative tumors, which are presumed to be related to rhabdoid tumors, may not be enrolled in the international trial, which addresses treatment of hepatoblastoma that includes small cell undifferentiated histology, hepatocellular carcinoma, and hepatic malignancy of childhood, not otherwise specified (NOS), but not rhabdoid tumor of the liver. In this trial, all patients with histology consistent with pure small cell undifferentiated hepatoblastoma, as assessed by the institutional pathologist, are required to have testing for SMARCB1 by immunohistochemistry according to the practices at the institution. In addition, presence of a blastemal component indicates conventional hepatoblastoma.[
A characteristic shared by both small cell undifferentiated hepatoblastoma and malignant rhabdoid tumor is the poor prognosis associated with each.[
- In 2009, the results of a study of 11 young children with low AFP levels and small cell morphology were reported. Ten children died of disease progression, and one child died of complications. Six of six children tested were SMARCB1 negative, but only one child had any rhabdoid morphology. This finding suggests that many or all liver tumors with small cell morphology and very low AFP levels in young children may be rhabdoid tumors of the liver. These tumors have a poor prognosis that is associated with the driver variant.[
84 ] - A single-institution study of seven children with small cell morphology liver tumors found that all retained expression of SMARCB1. Six children survived, and one child died of complications from liver transplant.[
88 ] - A study of 23 liver tumors from the Kiel tumor bank found 12 tumors with small cell morphology. Nine tumors had malignant rhabdoid tumor classic histology, and two tumors had mixed small cell and rhabdoid histologies. Outcomes were not provided, but it was noted that rhabdoid brain tumors had small cell, not classic, rhabdoid histology.[
89 ] - In a single-institution study of six children with SMARCB1-negative liver tumors, two children with small cell morphology died. The remaining four children with classic rhabdoid histology were not treated with cisplatin-based therapy; three children survived, and one child died of complications from transplant.[
90 ] - A report from the COG AHEP0731 (NCT00980460) trial identified 35 of 177 evaluable patients (19%) with small cell undifferentiated hepatoblastoma confirmed by central review.[
86 ] SMARCB1 nuclear expression was retained in 33 of 35 patients. Unlike previous reports, the presence of small cell undifferentiated histology did not correlate with age, sex, or AFP levels at diagnosis. The 5-year EFS rates for patients with low-, intermediate-, and high-risk small cell undifferentiated hepatoblastoma were 86% (95% confidence interval [CI], 33%–98%), 81% (95% CI, 51%–92%), and 29% (95% CI, 4%–81%), respectively. The 5-year EFS rates for patients with low-, intermediate-, and high-risk hepatoblastoma without small cell undifferentiated histology were 87% (95% CI, 72%–95%), 88% (95% CI, 79%–95%), and 55% (95% CI, 33%–74%); P = .17), respectively. In this trial, concordance between local and central review was poor, and they agreed in only 9 of 35 cases (26%). All tumors were tested for SMARCB1 expression by immunohistochemistry. In this study, hepatoblastoma that would otherwise be considered very low risk or low risk was upgraded to intermediate risk if any small cell undifferentiated elements were found. For more information, seeTable 5 .
The outcomes of the CHIC trial of childhood liver tumors may clarify some of the questions regarding these different histological and genetic findings.
Risk Stratification
There are significant differences among childhood cancer study groups in risk stratification used to determine treatment, making it difficult to compare results of the different treatments.
| | COG (AHEP-0731) | SIOPEL (SIOPEL-3, -3HR, -4, -6) | GPOH | JPLT (JPLT-2 and -3) |
|---|---|---|---|---|
| AFP = alpha-fetoprotein; COG = Children's Oncology Group; GPOH = Gesellschaft für Pädiatrische Onkologie und Hämatologie (Society for Paediatric Oncology and Haematology); JPLT = Japanese Study Group for Pediatric Liver Tumor; PRETEXT = PRE-Treatment EXTent of disease; SIOPEL = International Childhood Liver Tumors Strategy Group. | ||||
| a Adapted from Czauderna et al.[ |
||||
| b For more information about the annotations used in PRETEXT, see |
||||
| c The COG and PRETEXT definitions of vascular involvement differ. | ||||
| Very low risk | PRETEXT I or II; well-differentiated fetal histology; primary resection at diagnosis | |||
| Low risk/standard risk | PRETEXT I or II of any histology with primary resection at diagnosis | PRETEXT I, II, or III | PRETEXT I, II, or III | PRETEXT I, II, or III |
| Intermediate riskb | PRETEXT II, III, or IV unresectable at diagnosis; or V+c, P+, E+ | PRETEXT IV or any PRETEXT with rupture; or N1, P2, P2a, V3, V3a; or multifocal | ||
| High riskb | Any PRETEXT with M+; AFP level <100 ng/mL | Any PRETEXT; V+, P+, E+, M+; AFP level <100 ng/mL; tumor rupture | Any PRETEXT with V+, E+, P+, M+ or multifocal | Any PRETEXT with M1 or N2; or AFP level <100 ng/mL |
International risk classification model
The CHIC group developed a novel risk stratification system for use in international clinical trials on the basis of prognostic features present at diagnosis. CHIC unified the disparate definitions and staging systems used by pediatric cooperative multicenter trial groups, enabling the comparison of studies conducted by heterogeneous groups in different countries.[
Based on the initial univariate analysis of the data combined with historical clinical treatment patterns and data from previous large clinical trials, five backbone groups were selected, which allowed for further risk stratification. Subsequent multivariate analysis on the basis of these backbone groups defined the following clinical prognostic factors: PRETEXT group (I, II, III, or IV), presence of metastasis (yes or no), and AFP (≤100 ng/mL). The backbone groups are as follows:[
- Backbone 1: PRETEXT I/II, not metastatic, AFP greater than 100 ng/mL.
- Backbone 2: PRETEXT III, not metastatic, AFP greater than 100 ng/mL.
- Backbone 3: PRETEXT IV, not metastatic, AFP greater than 100 ng/mL.
- Backbone 4: Any PRETEXT group, metastatic disease at diagnosis, AFP greater than 100 ng/mL.
- Backbone 5: Any PRETEXT group, metastatic or not, AFP less than or equal to 100 ng/mL at diagnosis.
Other diagnostic factors (e.g., age) were queried for each of the backbone categories, including the presence of at least one of the following PRETEXT annotations (defined as VPEFR+, see
- V: Involvement of vena cava or all three hepatic veins, or both.
- P: Involvement of portal bifurcation or both right and left portal veins, or both.
- E: Extrahepatic contiguous tumor extension.
- F: Multifocal liver tumor.
- R: Tumor rupture at diagnosis.
An assessment of surgical resectability at diagnosis was added for PRETEXT I and II patients. Patients in each of the five backbone categories were stratified on the basis of backwards stepwise elimination multivariable analysis of additional patient characteristics, including age and presence or absence of PRETEXT annotation factors (V, P, E, F, and R). Each of these subcategories received one of four risk designations (very low, low, intermediate, or high). The result of the multivariate analysis was used to assign patients to very low-, low-, intermediate-, and high-risk categories, as shown in
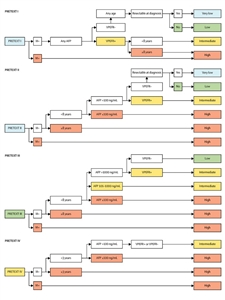
Figure 2. Risk stratification trees for the Children's Hepatic tumors International Collaboration—Hepatoblastoma Stratification (CHIC-HS). Very low-risk group and low-risk group are separated only by their resectability at diagnosis, which has been defined by international consensus as part of the surgical guidelines for the collaborative trial, Paediatric Hepatic International Tumour Trial (PHITT). Separate risk stratification trees are used for each of the four PRETEXT groups. AFP = alpha-fetoprotein. M = metastatic disease. PRETEXT = PRETreatment EXTent of disease. Reprinted from The Lancet Oncology, Volume 18, Meyers RL, Maibach R, Hiyama E, Häberle B, Krailo M, Rangaswami A, Aronson DC, Malogolowkin MH, Perilongo G, von Schweinitz D, Ansari M, Lopez-Terrada D, Tanaka Y, Alaggio R, Leuschner I, Hishiki T, Schmid I, Watanabe K, Yoshimura K, Feng Y, Rinaldi E, Saraceno D, Derosa M, Czauderna P, Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children's Hepatic tumors International Collaboration, Pages 122–131, Copyright (2017), with permission from Elsevier.
Treatment of Hepatoblastoma
Treatment options for newly diagnosed hepatoblastoma depend on the following:
- Whether the cancer is resectable at diagnosis.
- The tumor histology.
- How the cancer responds to chemotherapy.
- Whether the cancer has metastasized.
Cisplatin-based chemotherapy has resulted in a survival rate of more than 90% for children with PRETEXT and POST-Treatment EXTent (POSTTEXT) group I and II resectable disease before or after chemotherapy.[
Chemotherapy regimens used in the treatment of hepatoblastoma and their respective outcomes are described in
| Study | Chemotherapy Regimen | Number of Patients | Outcomes |
|---|---|---|---|
| AFP = alpha-fetoprotein; C5V = cisplatin, fluorouracil (5-FU), and vincristine; CARBO = carboplatin; CCG = Children's Cancer Group; CDDP = cisplatin; CITA = pirarubicin-cisplatin; COG = Children's Oncology Group; DOXO = doxorubicin; EFS = event-free survival; GPOH = Gesellschaft für Pädiatrische Onkologie und Hämatologie (Society for Paediatric Oncology and Haematology); H+ = rupture or intraperitoneal hemorrhage; HR = high risk; IFOS = ifosfamide; IPA = ifosfamide, cisplatin, and doxorubicin; ITEC = ifosfamide, pirarubicin, etoposide, and carboplatin; JPLT = Japanese Study Group for Pediatric Liver Tumor; LR = low risk; NR = not reported; OS = overall survival; PLADO = cisplatin and doxorubicin; POG = Pediatric Oncology Group; PRETEXT = PRE-Treatment EXTent of disease; SIOPEL = International Childhood Liver Tumors Strategy Group; SR = standard risk; SUPERPLADO = cisplatin, doxorubicin, and carboplatin; THP = tetrahydropyranyl-adriamycin (pirarubicin); VP = vinorelbine and cisplatin; VPE+ = venous, portal, and extrahepatic involvement; VP16 = etoposide. | |||
| a Adapted from Czauderna et al.,[ |
|||
| b Study closed early because of inferior results in the CDDP/CARBO arm. | |||
| INT0098 (CCG/POG) 1989–1992 | C5V vs. CDDP/DOXO | Stage I/II: 50 | 4-Year EFS/OS: |
| I/II = 88%/100% vs. 96%/96% | |||
| Stage III: 83 | III = 60%/68% vs. 68%/71% | ||
| Stage IV: 40 | IV = 14%/33% vs. 37%/42% | ||
| P9645 (COG) b 1999–2002 | C5V vs. CDDP/CARBO | Stage III: 38 | 3-year EFS/OS: |
| III/IV: C5V = 60%/74%; CDDP/CARBO = 38%/54% | |||
| Stage IV: 50 | |||
| AHEP0731 (COG) 2010–2014[ |
LR: C5V (2 cycles) | LR (stage I/II): 49 | 5-year EFS: 88%;5-year OS: 91% |
| HB 94 (GPOH) 1994–1997 | I/II: IFOS/CDDP/DOXO | Stage I: 27 | 4-Year EFS/OS: |
| I = 89%/96% | |||
| Stage II: 3 | II = 100%/100% | ||
| III/IV: IFOS/CDDP/DOXO + VP/CARBO | Stage III: 25 | III = 68%/76% | |
| Stage IV: 14 | IV = 21%/36% | ||
| HB 99 (GPOH) 1999–2004 | SR: IPA | SR: 58 | 3-Year EFS/OS: |
| SR = 90%/88% | |||
| HR: CARBO/VP16 | HR: 42 | HR = 52%/55% | |
| SIOPEL-2 1994–1998 | SR: PLADO | PRETEXT I: 6 | 3-Year EFS/OS: |
| SR: 73%/91% | |||
| PRETEXT II: 36 | |||
| PRETEXT III: 25 | |||
| HR: CDDP/CARBO/DOXO | PRETEXT IV: 21 | HR: IV = 48%/61% | |
| Metastases: 25 | HR: metastases = 36%/44% | ||
| SIOPEL-3 1998–2006 | SR: CDDP vs. PLADO | SR: PRETEXT I: 18 | 3-Year EFS/OS: |
| SR: CDDP = 83%/95%; PLADO = 85%/93% | |||
| PRETEXT II: 133 | |||
| PRETEXT III: 104 | |||
| HR: SUPERPLADO | HR: PRETEXT IV: 74 | HR: Overall = 65%/69% | |
| VPE+: 70 | |||
| Metastases: 70 | Metastases = 57%/63% | ||
| AFP <100 ng/mL: 12 | |||
| SIOPEL-4 2005–2009 | HR: Block A: Weekly; CDDP/3 weekly DOXO; Block B: CARBO/DOXO | PRETEXT I: 2 | 3-Year EFS/OS: |
| All HR = 76%/83% | |||
| PRETEXT II: 17 | |||
| PRETEXT III: 27 | |||
| PRETEXT IV: 16 | HR: IV = 75%/88% | ||
| Metastases: 39 | HR: Metastases = 77%/79% | ||
| JPLT-1 1991–1999 | I/II: CDDP(30)/THP-DOXO | Stage I: 9 | 5-Year EFS/OS: |
| I = NR/100% | |||
| Stage II: 32 | II = NR/76% | ||
| III/IV: CDDP(60)/THP-DOXO | Stage IIIa: 48 | IIIa = NR/50% | |
| Stage IIIb: 25 | IIIb = NR/64% | ||
| Stage IV: 20 | IV = NR/77% | ||
| JPLT-2 1999–2010[ |
Initial surgery and 2 cycles of CITA | Stratum 1: PRETEXT I/II, 0 annotation factors except H+ (n = 40) | 5-Year EFS/OS: |
| 74.2%/89.9% | |||
| 2 cycles of CITA followed by surgery and 2–4 cycles of CITA | Stratum 2: PRETEXT II with multifocality (n = 80) | 84.8%/90.8% | |
| 2 cycles of CITA followed by 2 cycles of CITA (responders); attempted surgery including transplant | Stratum 3: PRETEXT I/II (annotation factors present) and III/IV (n = 176) responders | 71.6%/85.9% | |
| 2 cycles of CITA followed by 2 cycles of ITEC (nonresponders); attempted surgery including transplant | Stratum 4: PRETEXT I/II (annotation factors present) and III/IV (n = 59) nonresponders | 59.1%/67.3% | |
Treatment options for hepatoblastoma that is resectable at diagnosis
Approximately 20% to 30% of children with hepatoblastoma have resectable disease at diagnosis. COG surgical guidelines (AHEP0731 [NCT00980460] appendix) recommend tumor resection at diagnosis without preoperative chemotherapy in children with PRETEXT I tumors and PRETEXT II tumors with greater than 1 cm radiographic margin on the vena cava and middle hepatic and portal veins. Outcomes for patients after undergoing a complete resection at diagnosis, compared with patients who had positive microscopic margins found at resection, are similar after receiving chemotherapy.[
Prognosis varies depending on the histological subtype, as follows:
- Patients with well-differentiated fetal histology (4% of hepatoblastomas) have a 3- to 5-year OS rate of 100% with minimal or no adjuvant chemotherapy.[
50 ,51 ,52 ,96 ] - Patients with non–well-differentiated fetal histology, non–small cell undifferentiated hepatoblastomas have a 3- to 4-year OS rate of 90% to 100% with adjuvant chemotherapy.[
50 ,51 ,54 ,56 ,97 ] - If any small cell undifferentiated elements are present, the 3-year survival rate is 40% to 70%.[
50 ,53 ]
Treatment options for hepatoblastoma resectable at diagnosis showing non–well-differentiated fetal histology include the following:
- Resection followed by two to four cycles of chemotherapy.[
58 ]
Re-resection of positive microscopic margins may not be necessary. Conclusive evidence is lacking for tumors with resection at diagnosis compared with those with positive microscopic margins resected after preoperative chemotherapy.
Evidence (gross surgical resection, with or without microscopic margins, and postoperative chemotherapy):
- In the COG AHEP0731 (NCT00980460) trial, 49 of 51 patients with stage I or stage II hepatoblastoma (without pure fetal histology) received two cycles of adjuvant chemotherapy consisting of cisplatin, fluorouracil, and vincristine.[
93 ][Level of evidence C1]- The 5-year EFS rate was 88%, and the 5-year OS rate was 91%.
- This outcome is comparable to the outcomes for children treated with four cycles after initial resection, and to the outcomes for children treated with two cycles of neoadjuvant chemotherapy before resection followed by two cycles of chemotherapy after resection.
- There is no reliable data for local recurrence risk in patients with a positive microscopic margin status who underwent resection at diagnosis.[
68 ] SIOPEL studies suggest that in patients who received preoperative chemotherapy, positive microscopic margin did not increase risk of local recurrence.[56 ,57 ,73 ]; [95 ][Level of evidence C1]- In a European study conducted between 1990 and 1994, 11 patients had tumor found at the surgical margins after hepatic resection and two patients died, neither of whom had a local recurrence. None of the 11 patients underwent a second resection, and only one patient received radiation therapy postoperatively. All of the patients were treated with four courses of cisplatin and doxorubicin before surgery and received two courses of postoperative chemotherapy.[
73 ] - In another European study of high-risk hepatoblastoma, 11 patients had microscopic residual tumor remaining after initial surgery and received two to four postoperative cycles of chemotherapy with no additional surgery. Of these 11 patients, 9 survived.[
57 ] - In the SIOPEL-2 study, 13 of 13 patients with microscopic positive resection margins survived.[
56 ] - An unplanned retrospective study of the SIOPEL-2 and SIOPEL-3 trials found that after four courses of cisplatin for standard-risk patients and seven courses of cisplatin alternating with doxorubicin/carboplatin for high-risk patients, resection was performed where imaging suggested it would be safe. Of the 431 children treated in these trials, 58 patients had positive microscopic tumor margins, and 371 patients were in complete remission. There were no statistically significant differences in the rates of local recurrence, EFS, or OS between the two groups.[
95 ][Level of evidence C1]
- In a European study conducted between 1990 and 1994, 11 patients had tumor found at the surgical margins after hepatic resection and two patients died, neither of whom had a local recurrence. None of the 11 patients underwent a second resection, and only one patient received radiation therapy postoperatively. All of the patients were treated with four courses of cisplatin and doxorubicin before surgery and received two courses of postoperative chemotherapy.[
- A randomized clinical trial demonstrated comparable efficacy with postoperative cisplatin/vincristine/fluorouracil and cisplatin/doxorubicin in the treatment of patients with hepatoblastoma.[
51 ]- Although survival outcomes were nominally higher for the children who received cisplatin/doxorubicin, this difference was not statistically significant.
- The combination of cisplatin/vincristine/fluorouracil was significantly less toxic than were the doses of cisplatin/doxorubicin.
Results of chemotherapy clinical trials are described in
Treatment options for hepatoblastoma of well-differentiated fetal (pure fetal) histology resectable at diagnosis include the following:
- Complete surgical resection followed by watchful waiting or chemotherapy.[
52 ]
Evidence (complete surgical resection followed by watchful waiting or chemotherapy):
- In a COG prospective clinical trial (INT0098), nine children with stage I (completely resected) well-differentiated fetal histology and fewer than two mitoses per high-power field were treated with four cycles of adjuvant doxorubicin.[
51 ]- At a median follow-up of 5.1 years, the EFS and OS rates were 100% for all nine children.
- In the COG P9645 (NCT00003994) study, 16 patients with stage I (completely resected) tumors had well-differentiated fetal histology and received no adjuvant chemotherapy. In a retrospective PRETEXT classification of 21 of these 25 patients with adequate data, PRETEXT I, II, and III tumors were found in 7, 10, and 4 patients, respectively.[
52 ]- The EFS and OS rates were 100% for patients with stage I well-differentiated fetal histology, including one patient who had a second surgery to address a positive tumor margin.
Treatment options for hepatoblastoma that is not resectable or not resected at diagnosis
Approximately 70% to 80% of children with hepatoblastoma have tumors that are not resected at diagnosis. COG surgical guidelines (AHEP0731 [NCT00980460] appendix) recommend a diagnostic biopsy without an attempt to resect the tumor in children with PRETEXT II tumors with less than 1-cm radiographic margin on the vena cava and middle hepatic vein and in all children with PRETEXT III and IV tumors.
Treatment options for hepatoblastoma that is not resectable or is not resected at diagnosis include the following:
- Chemotherapy followed by reassessment of surgical resectability and complete surgical resection.
- Chemotherapy followed by reassessment of surgical resectability and orthotopic liver transplant.[
54 ,71 ,98 ,99 ,100 ,101 ,102 ,103 ] - Transarterial chemoembolization (TACE) and transarterial radioembolization (TARE). TACE and TARE may be used to improve resectability before definitive surgical approaches.[
104 ,105 ,106 ]
Tumor rupture at presentation, resulting in major hemorrhage that can be controlled by transcatheter arterial embolization or partial resection to stabilize the patient, does not preclude a favorable outcome when followed by chemotherapy and definitive surgery.[
In recent years, most children with hepatoblastoma have been treated with chemotherapy. In European cancer centers, children with resectable hepatoblastoma at diagnosis are treated with preoperative chemotherapy, which may reduce the incidence of surgical complications at the time of resection.[
Patients whose tumors remain unresectable after chemotherapy should consider a liver transplant.[
Evidence (chemotherapy followed by reassessment of surgical resectability and complete surgical resection or liver transplant):
- In the SIOPEL-1 study, preoperative chemotherapy (doxorubicin and cisplatin) was given to all children with hepatoblastoma with or without metastases. After chemotherapy, and excluding those who underwent a liver transplant (<5% of patients), complete resection was performed.[
54 ]- The chemotherapy was well tolerated.
- Complete resection was obtained in 87% of children.
- This strategy resulted in an OS rate of 75% at 5 years after diagnosis.
- Identical results were seen in a follow-up international study (SIOPEL-2).[
56 ] - The SIOPEL-3 study compared cisplatin alone with cisplatin and doxorubicin in patients with preoperative standard-risk hepatoblastoma. Standard risk was defined as tumor confined to the liver and involving as many as three sectors.[
97 ][Level of evidence A1]- The resection rates and OS rates were similar for the cisplatin (95%) and cisplatin/doxorubicin (93%) groups.
- In a pilot study, SIOPEL-3HR, cisplatin alternating with carboplatin/doxorubicin was administered in a dose-intensive fashion to high-risk patients with hepatoblastoma.[
57 ]- In 74 patients with PRETEXT IV tumors, 22 of whom also had metastases, 31 patients had tumors that became resectable, and 26 patients underwent transplant. The 3-year OS rate was 69% (± 11%).
- Of the 70 patients with metastases enrolled in the trial, the 3-year EFS rate was 56%, and the OS rate was 62%. Of patients with lung metastases, 50% were able to achieve complete remission of metastases with chemotherapy alone (without lung surgery).
- SIOPEL-4 (NCT00077389) was a multinational feasibility trial of dose-dense cisplatin/doxorubicin chemotherapy and radical surgery for a group of children with high-risk hepatoblastoma. Surgical removal of all remaining tumor lesions after chemotherapy was performed if feasible (including liver transplant and metastasectomy, if needed). Patients who underwent liver resection or liver transplant after three cycles of chemotherapy subsequently received two postoperative cycles of carboplatin and doxorubicin. Patients whose tumors remained unresectable after three cycles of chemotherapy received two cycles of very intensive carboplatin and doxorubicin before surgery. The primary tumor masses were identified as PRETEXT groups II (27%), III (44%), and IV (26%).[
74 ][Level of evidence B4]- Ninety-seven percent of patients (60 of 61) had a partial response with chemotherapy.
- Eighty-five percent of patients (53) underwent complete macroscopic resection; tumor was microscopically present in five patients, all of whom are disease-free survivors.
- Two patients died postoperatively.
- There were 37 partial hepatectomies and 16 liver transplants.
- The study had a total of 62 high-risk patients; 74% of patients (62%–84%) underwent resection.
- The 3-year disease-free survival (DFS) rate was 76% (95% CI, 65%–87%).
- The 3-year OS rate was 83% (95% CI, 73%–93%).
- Of the 16 patients with PRETEXT IV tumors, 11 were downstaged after chemotherapy—6 patients to PRETEXT group III, 4 patients to PRETEXT group II, and 1 patient to PRETEXT group I. Twelve tumors became resectable; subsequently, four patients underwent a partial hepatectomy and eight patients underwent a liver transplant. For patients who presented with PRETEXT IV disease:
- The 3-year DFS rate was 73% (95% CI, 51%–96%).
- The 3-year OS rate was 80% (95% CI, 60%–100%).
- In approximately 75% of children and adolescents with initially unresectable hepatoblastoma, tumors can be rendered resectable with cisplatin-based preoperative chemotherapy, and 60% to 65% of patients will survive disease-free.[
108 ]
In the United States, patients with unresectable tumors have been treated with chemotherapy before resection or transplant.[
The COG conducted a single-arm phase III trial (AHEP0731 [NCT00980460]) for patients with intermediate-risk hepatoblastoma. The study included 93 patients with unresectable nonmetastatic disease and 9 patients with a complete resection at diagnosis. All of the tumors had small cell undifferentiated histology. The addition of doxorubicin to standard treatment (cisplatin, fluorouracil, and vincristine) was assessed for feasibility and efficacy. In the 93 patients with initially unresectable disease, the 5-year EFS rate was 85% (95% CI, 79%–93%), and the OS rate was 95% (95% CI, 87%–98%).[
Chemotherapy followed by TACE, then high-intensity focused ultrasound, showed promising results in China for patients with PRETEXT III and IV tumors, some of which were resectable. Patients did not undergo surgical resection because of parent refusal.[
Treatment options for hepatoblastoma with metastases at diagnosis
The outcomes of patients with metastatic hepatoblastoma at diagnosis are poor, but long-term survival and cure are possible.[
Treatment options for hepatoblastoma with metastases at diagnosis include the following:
- Chemotherapy followed by reassessment of surgical resectability.
- If the primary tumor and extrahepatic disease (usually pulmonary nodules) are resectable after chemotherapy, surgical resection is followed by additional chemotherapy.
- If extrahepatic metastatic disease is in complete remission after chemotherapy and/or surgical resection of lung nodule but the primary tumor remains unresectable, orthotopic liver transplant is warranted.
- If extrahepatic metastatic disease is not resectable or the patient is not a transplant candidate, additional chemotherapy, TACE, TARE, or radiation therapy may be indicated.[
106 ]
The standard combination chemotherapy regimen in North America is four courses of cisplatin/vincristine/fluorouracil [
High-dose chemotherapy with stem cell rescue does not appear to be more effective than standard multiagent chemotherapy.[
Evidence (chemotherapy followed by surgery to treat metastatic disease at diagnosis):
- A subset of 39 patients presenting with metastases were enrolled in the SIOPEL-4 (NCT00077389) trial, a multinational feasibility trial of dose-dense cisplatin/doxorubicin chemotherapy and radical surgery for a group of children with high-risk hepatoblastoma. Patients who underwent liver resection or transplant after three cycles of chemotherapy subsequently received two postoperative cycles of carboplatin and doxorubicin. Patients whose tumors were unresectable after three cycles of chemotherapy received two additional cycles of very intensive carboplatin and doxorubicin before surgery.[
74 ][Level of evidence B4]- After three cycles of chemotherapy, there was a complete response (only in the metastases) in 20 of 39 patients and a partial response in 18 of 39 patients. Nineteen of the patients who achieved a complete response were alive without disease 3 years after diagnosis.
- Of the patients who achieved a partial response, seven patients underwent metastasectomy near the time of resection or liver transplant, with an OS rate of 100%. An additional seven patients with residual small pulmonary nodules underwent resection without metastasectomy; of those, six patients did well and one patient had a recurrence.
- Two patients with initial metastases eventually experienced a recurrence.
- Liver transplant, rather than resection alone, was needed to treat 7 of the 39 patients who presented with metastases.
- For the subset of 39 patients presenting with metastases, the 3-year DFS rate was 77% (95% CI, 63%–90%), and the OS rate was 79% (95% CI, 66%–92%).
In patients with resected primary tumors, any remaining pulmonary metastases should be surgically removed, if possible.[
If extrahepatic disease is in complete remission after chemotherapy, and the primary tumor remains unresectable, an orthotopic liver transplant may be performed.[
The outcome results are discrepant for patients with lung metastases at diagnosis who undergo orthotopic liver transplant after complete resolution of lung disease in response to pretransplant chemotherapy. Some studies have reported favorable outcomes for these patients,[
If extrahepatic disease is not resectable after chemotherapy or the patient is not a transplant candidate, alternative treatment approaches include the following:
- Other chemotherapy agents. Chemotherapy agents such as irinotecan, high-dose cisplatin/etoposide, or continuous-infusion doxorubicin have been used.[
120 ,121 ,122 ]; [123 ][Level of evidence C1] - TACE.[
105 ,124 ] - Radiation therapy.[
125 ]
Treatment of Progressive or Recurrent Hepatoblastoma
The prognosis for a patient with progressive or recurrent hepatoblastoma depends on several factors, including the following:[
- Site of recurrence.
- Previous treatment.
- Individual patient considerations.
Treatment options for progressive or recurrent hepatoblastoma include the following:
- Surgical resection. In patients with hepatoblastoma that is completely resected at initial diagnosis, aggressive surgical treatment of isolated pulmonary metastases that develop in the course of the disease, in conjunction with an overall strategy that includes chemotherapy, may make extended DFS possible.[
117 ,126 ,127 ]If possible, isolated metastases are resected completely in patients whose primary tumor is controlled.[
128 ] A retrospective analysis of patients in the SIOPEL 1, 2, and 3 studies showed a 12% incidence of recurrence after complete remission by imaging and AFP levels. Outcome after recurrence was best if the tumor was amenable to surgery. Of patients who underwent chemotherapy and surgery, the 3-year EFS rate was 34%, and the OS rate was 43%.[126 ][Level of evidence C1]If all of the recurrent disease cannot be surgically removed, patients should consider enrolling in a clinical trial. Phase I and phase II clinical trials may be appropriate.
- Chemotherapy. Analysis of survival after recurrence demonstrated that some patients treated with cisplatin/vincristine/fluorouracil could be salvaged with doxorubicin-containing regimens, but patients treated with doxorubicin/cisplatin could not be salvaged with vincristine/fluorouracil.[
129 ] The addition of doxorubicin to vincristine/fluorouracil/cisplatin was clinically evaluated in the COG study AHEP0731 (NCT00980460).Combined vincristine/irinotecan and single-agent irinotecan have been used with some success.[
123 ]; [122 ][Level of evidence C1]A review of COG phase I and II studies found no promising agents for relapsed hepatoblastoma.[
130 ] - Liver transplant. Liver transplant should be considered for patients with nonmetastatic disease recurrence in the liver that is not amenable to resection.[
71 ,98 ,101 ] - Percutaneous ablation. Percutaneous radiofrequency ablation has been used as an alternative to surgical resection of oligometastatic hepatoblastoma.[
131 ][Level of evidence C1] Percutaneous ablation techniques may also be considered for palliation.[132 ]
Treatment Options Under Clinical Evaluation for Hepatoblastoma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the
References:
- Childhood cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). National Cancer Institute, 2012, Section 29.
Also available online . Last accessed August 11, 2022. - Bulterys M, Goodman MT, Smith MA, et al.: Hepatic tumors. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649, pp 91-98.
Also available online . Last accessed August 11, 2022. - National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute.
Available online . Last accessed February 25, 2025. - Turcotte LM, Georgieff MK, Ross JA, et al.: Neonatal medical exposures and characteristics of low birth weight hepatoblastoma cases: a report from the Children's Oncology Group. Pediatr Blood Cancer 61 (11): 2018-23, 2014.
- Tanimura M, Matsui I, Abe J, et al.: Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res 58 (14): 3032-5, 1998.
- McLaughlin CC, Baptiste MS, Schymura MJ, et al.: Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol 163 (9): 818-28, 2006.
- Darbari A, Sabin KM, Shapiro CN, et al.: Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 38 (3): 560-6, 2003.
- Kamien BA, Gabbett MT: Aicardi syndrome associated with hepatoblastoma and pulmonary sequestration. Am J Med Genet A 149A (8): 1850-2, 2009.
- DeBaun MR, Tucker MA: Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J Pediatr 132 (3 Pt 1): 398-400, 1998.
- Weksberg R, Shuman C, Smith AC: Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet 137C (1): 12-23, 2005.
- Iwama T, Mishima Y: Mortality in young first-degree relatives of patients with familial adenomatous polyposis. Cancer 73 (8): 2065-8, 1994.
- Garber JE, Li FP, Kingston JE, et al.: Hepatoblastoma and familial adenomatous polyposis. J Natl Cancer Inst 80 (20): 1626-8, 1988.
- Giardiello FM, Petersen GM, Brensinger JD, et al.: Hepatoblastoma and APC gene mutation in familial adenomatous polyposis. Gut 39 (96): 867-9, 1996.
- Ito E, Sato Y, Kawauchi K, et al.: Type 1a glycogen storage disease with hepatoblastoma in siblings. Cancer 59 (10): 1776-80, 1987.
- Ikeda H, Hachitanda Y, Tanimura M, et al.: Development of unfavorable hepatoblastoma in children of very low birth weight: results of a surgical and pathologic review. Cancer 82 (9): 1789-96, 1998.
- Spector LG, Birch J: The epidemiology of hepatoblastoma. Pediatr Blood Cancer 59 (5): 776-9, 2012.
- Buonuomo PS, Ruggiero A, Vasta I, et al.: Second case of hepatoblastoma in a young patient with Simpson-Golabi-Behmel syndrome. Pediatr Hematol Oncol 22 (7): 623-8, 2005 Oct-Nov.
- Tan ZH, Lai A, Chen CK, et al.: Association of trisomy 18 with hepatoblastoma and its implications. Eur J Pediatr 173 (12): 1595-8, 2014.
- Trobaugh-Lotrario AD, Venkatramani R, Feusner JH: Hepatoblastoma in children with Beckwith-Wiedemann syndrome: does it warrant different treatment? J Pediatr Hematol Oncol 36 (5): 369-73, 2014.
- Hoyme HE, Seaver LH, Jones KL, et al.: Isolated hemihyperplasia (hemihypertrophy): report of a prospective multicenter study of the incidence of neoplasia and review. Am J Med Genet 79 (4): 274-8, 1998.
- Clericuzio CL, Martin RA: Diagnostic criteria and tumor screening for individuals with isolated hemihyperplasia. Genet Med 11 (3): 220-2, 2009.
- Algar EM, St Heaps L, Darmanian A, et al.: Paternally inherited submicroscopic duplication at 11p15.5 implicates insulin-like growth factor II in overgrowth and Wilms' tumorigenesis. Cancer Res 67 (5): 2360-5, 2007.
- Steenman M, Westerveld A, Mannens M: Genetics of Beckwith-Wiedemann syndrome-associated tumors: common genetic pathways. Genes Chromosomes Cancer 28 (1): 1-13, 2000.
- Albrecht S, Hartmann W, Houshdaran F, et al.: Allelic loss but absence of mutations in the polyspecific transporter gene BWR1A on 11p15.5 in hepatoblastoma. Int J Cancer 111 (4): 627-32, 2004.
- Clericuzio CL, Chen E, McNeil DE, et al.: Serum alpha-fetoprotein screening for hepatoblastoma in children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia. J Pediatr 143 (2): 270-2, 2003.
- Mussa A, Molinatto C, Baldassarre G, et al.: Cancer Risk in Beckwith-Wiedemann Syndrome: A Systematic Review and Meta-Analysis Outlining a Novel (Epi)Genotype Specific Histotype Targeted Screening Protocol. J Pediatr 176: 142-149.e1, 2016.
- Aretz S, Koch A, Uhlhaas S, et al.: Should children at risk for familial adenomatous polyposis be screened for hepatoblastoma and children with apparently sporadic hepatoblastoma be screened for APC germline mutations? Pediatr Blood Cancer 47 (6): 811-8, 2006.
- Hirschman BA, Pollock BH, Tomlinson GE: The spectrum of APC mutations in children with hepatoblastoma from familial adenomatous polyposis kindreds. J Pediatr 147 (2): 263-6, 2005.
- Koch A, Denkhaus D, Albrecht S, et al.: Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res 59 (2): 269-73, 1999.
- Kalish JM, Doros L, Helman LJ, et al.: Surveillance Recommendations for Children with Overgrowth Syndromes and Predisposition to Wilms Tumors and Hepatoblastoma. Clin Cancer Res 23 (13): e115-e122, 2017.
- Eichenmüller M, Trippel F, Kreuder M, et al.: The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J Hepatol 61 (6): 1312-20, 2014.
- Jia D, Dong R, Jing Y, et al.: Exome sequencing of hepatoblastoma reveals novel mutations and cancer genes in the Wnt pathway and ubiquitin ligase complex. Hepatology 60 (5): 1686-96, 2014.
- Sumazin P, Chen Y, Treviño LR, et al.: Genomic analysis of hepatoblastoma identifies distinct molecular and prognostic subgroups. Hepatology 65 (1): 104-121, 2017.
- Carrillo-Reixach J, Torrens L, Simon-Coma M, et al.: Epigenetic footprint enables molecular risk stratification of hepatoblastoma with clinical implications. J Hepatol 73 (2): 328-341, 2020.
- Gröbner SN, Worst BC, Weischenfeldt J, et al.: The landscape of genomic alterations across childhood cancers. Nature 555 (7696): 321-327, 2018.
- Sekiguchi M, Seki M, Kawai T, et al.: Integrated multiomics analysis of hepatoblastoma unravels its heterogeneity and provides novel druggable targets. NPJ Precis Oncol 4: 20, 2020.
- Cairo S, Armengol C, Maibach R, et al.: A combined clinical and biological risk classification improves prediction of outcome in hepatoblastoma patients. Eur J Cancer 141: 30-39, 2020.
- Cancer Genome Atlas Research Network. Electronic address: wheeler@bcm.edu, Cancer Genome Atlas Research Network: Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 169 (7): 1327-1341.e23, 2017.
- Albrecht S, von Schweinitz D, Waha A, et al.: Loss of maternal alleles on chromosome arm 11p in hepatoblastoma. Cancer Res 54 (19): 5041-4, 1994.
- Cairo S, Armengol C, De Reyniès A, et al.: Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell 14 (6): 471-84, 2008.
- Yoon JM, Burns RC, Malogolowkin MH, et al.: Treatment of infantile choriocarcinoma of the liver. Pediatr Blood Cancer 49 (1): 99-102, 2007.
- Boman F, Bossard C, Fabre M, et al.: Mesenchymal hamartomas of the liver may be associated with increased serum alpha foetoprotein concentrations and mimic hepatoblastomas. Eur J Pediatr Surg 14 (1): 63-6, 2004.
- Blohm ME, Vesterling-Hörner D, Calaminus G, et al.: Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr Hematol Oncol 15 (2): 135-42, 1998 Mar-Apr.
- Wu JT, Book L, Sudar K: Serum alpha fetoprotein (AFP) levels in normal infants. Pediatr Res 15 (1): 50-2, 1981.
- Schneider DT, Calaminus G, Göbel U: Diagnostic value of alpha 1-fetoprotein and beta-human chorionic gonadotropin in infancy and childhood. Pediatr Hematol Oncol 18 (1): 11-26, 2001 Jan-Feb.
- Nakagawara A, Ikeda K, Tsuneyoshi M, et al.: Hepatoblastoma producing both alpha-fetoprotein and human chorionic gonadotropin. Clinicopathologic analysis of four cases and a review of the literature. Cancer 56 (7): 1636-42, 1985.
- Perilongo G, Malogolowkin M, Feusner J: Hepatoblastoma clinical research: lessons learned and future challenges. Pediatr Blood Cancer 59 (5): 818-21, 2012.
- Trobaugh-Lotrario AD, Katzenstein HM: Chemotherapeutic approaches for newly diagnosed hepatoblastoma: past, present, and future strategies. Pediatr Blood Cancer 59 (5): 809-12, 2012.
- Trobaugh-Lotrario AD, Chaiyachati BH, Meyers RL, et al.: Outcomes for patients with congenital hepatoblastoma. Pediatr Blood Cancer 60 (11): 1817-25, 2013.
- Meyers RL, Rowland JR, Krailo M, et al.: Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 53 (6): 1016-22, 2009.
- Ortega JA, Douglass EC, Feusner JH, et al.: Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol 18 (14): 2665-75, 2000.
- Malogolowkin MH, Katzenstein HM, Meyers RL, et al.: Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children's Oncology Group. J Clin Oncol 29 (24): 3301-6, 2011.
- De Ioris M, Brugieres L, Zimmermann A, et al.: Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: the SIOPEL group experience. Eur J Cancer 44 (4): 545-50, 2008.
- Pritchard J, Brown J, Shafford E, et al.: Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach--results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol 18 (22): 3819-28, 2000.
- Perilongo G, Brown J, Shafford E, et al.: Hepatoblastoma presenting with lung metastases: treatment results of the first cooperative, prospective study of the International Society of Paediatric Oncology on childhood liver tumors. Cancer 89 (8): 1845-53, 2000.
- Perilongo G, Shafford E, Maibach R, et al.: Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology--SIOPEL 2. Eur J Cancer 40 (3): 411-21, 2004.
- Zsíros J, Maibach R, Shafford E, et al.: Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol 28 (15): 2584-90, 2010.
- Czauderna P, Haeberle B, Hiyama E, et al.: The Children's Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer 52: 92-101, 2016.
- Katzenstein HM, Furman WL, Malogolowkin MH, et al.: Upfront window vincristine/irinotecan treatment of high-risk hepatoblastoma: A report from the Children's Oncology Group AHEP0731 study committee. Cancer 123 (12): 2360-2367, 2017.
- O'Neill AF, Towbin AJ, Krailo MD, et al.: Characterization of Pulmonary Metastases in Children With Hepatoblastoma Treated on Children's Oncology Group Protocol AHEP0731 (The Treatment of Children With All Stages of Hepatoblastoma): A Report From the Children's Oncology Group. J Clin Oncol 35 (30): 3465-3473, 2017.
- Fuchs J, Rydzynski J, Von Schweinitz D, et al.: Pretreatment prognostic factors and treatment results in children with hepatoblastoma: a report from the German Cooperative Pediatric Liver Tumor Study HB 94. Cancer 95 (1): 172-82, 2002.
- Aronson DC, Schnater JM, Staalman CR, et al.: Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol 23 (6): 1245-52, 2005.
- Meyers RL, Maibach R, Hiyama E, et al.: Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children's Hepatic tumors International Collaboration. Lancet Oncol 18 (1): 122-131, 2017.
- Haeberle B, Rangaswami A, Krailo M, et al.: The importance of age as prognostic factor for the outcome of patients with hepatoblastoma: Analysis from the Children's Hepatic tumors International Collaboration (CHIC) database. Pediatr Blood Cancer 67 (8): e28350, 2020.
- Dall'Igna P, Brugieres L, Christin AS, et al.: Hepatoblastoma in children aged less than six months at diagnosis: A report from the SIOPEL group. Pediatr Blood Cancer 65 (1): , 2018.
- Ortega JA, Krailo MD, Haas JE, et al.: Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: a report from the Childrens Cancer Study Group. J Clin Oncol 9 (12): 2167-76, 1991.
- Douglass EC, Reynolds M, Finegold M, et al.: Cisplatin, vincristine, and fluorouracil therapy for hepatoblastoma: a Pediatric Oncology Group study. J Clin Oncol 11 (1): 96-9, 1993.
- Meyers RL, Czauderna P, Otte JB: Surgical treatment of hepatoblastoma. Pediatr Blood Cancer 59 (5): 800-8, 2012.
- Hiyama E, Hishiki T, Watanabe K, et al.: Resectability and tumor response after preoperative chemotherapy in hepatoblastoma treated by the Japanese Study Group for Pediatric Liver Tumor (JPLT)-2 protocol. J Pediatr Surg 51 (12): 2053-2057, 2016.
- Becker K, Furch C, Schmid I, et al.: Impact of postoperative complications on overall survival of patients with hepatoblastoma. Pediatr Blood Cancer 62 (1): 24-8, 2015.
- Otte JB, Pritchard J, Aronson DC, et al.: Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer 42 (1): 74-83, 2004.
- McAteer JP, Goldin AB, Healey PJ, et al.: Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatr Transplant 17 (8): 744-50, 2013.
- Schnater JM, Aronson DC, Plaschkes J, et al.: Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer 94 (4): 1111-20, 2002.
- Zsiros J, Brugieres L, Brock P, et al.: Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol 14 (9): 834-42, 2013.
- Van Tornout JM, Buckley JD, Quinn JJ, et al.: Timing and magnitude of decline in alpha-fetoprotein levels in treated children with unresectable or metastatic hepatoblastoma are predictors of outcome: a report from the Children's Cancer Group. J Clin Oncol 15 (3): 1190-7, 1997.
- Nguyen R, McCarville MB, Sykes A, et al.: Rapid decrease of serum alpha-fetoprotein and tumor volume predicts outcome in children with hepatoblastoma treated with neoadjuvant chemotherapy. Int J Clin Oncol 23 (5): 900-907, 2018.
- Brown J, Perilongo G, Shafford E, et al.: Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 36 (11): 1418-25, 2000.
- D'Antiga L, Vallortigara F, Cillo U, et al.: Features predicting unresectability in hepatoblastoma. Cancer 110 (5): 1050-8, 2007.
- Czauderna P, Lopez-Terrada D, Hiyama E, et al.: Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr 26 (1): 19-28, 2014.
- López-Terrada D, Alaggio R, de Dávila MT, et al.: Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 27 (3): 472-91, 2014.
- Weinberg AG, Finegold MJ: Primary hepatic tumors of childhood. Hum Pathol 14 (6): 512-37, 1983.
- Haas JE, Muczynski KA, Krailo M, et al.: Histopathology and prognosis in childhood hepatoblastoma and hepatocarcinoma. Cancer 64 (5): 1082-95, 1989.
- Rowland JM: Hepatoblastoma: assessment of criteria for histologic classification. Med Pediatr Oncol 39 (5): 478-83, 2002.
- Trobaugh-Lotrario AD, Tomlinson GE, Finegold MJ, et al.: Small cell undifferentiated variant of hepatoblastoma: adverse clinical and molecular features similar to rhabdoid tumors. Pediatr Blood Cancer 52 (3): 328-34, 2009.
- Gunawan B, Schäfer KL, Sattler B, et al.: Undifferentiated small cell hepatoblastoma with a chromosomal translocation t(22;22)(q11;q13). Histopathology 40 (5): 485-7, 2002.
- Trobaugh-Lotrario A, Katzenstein HM, Ranganathan S, et al.: Small Cell Undifferentiated Histology Does Not Adversely Affect Outcome in Hepatoblastoma: A Report From the Children's Oncology Group (COG) AHEP0731 Study Committee. J Clin Oncol 40 (5): 459-467, 2022.
- Conran RM, Hitchcock CL, Waclawiw MA, et al.: Hepatoblastoma: the prognostic significance of histologic type. Pediatr Pathol 12 (2): 167-83, 1992 Mar-Apr.
- Zhou S, Gomulia E, Mascarenhas L, et al.: Is INI1-retained small cell undifferentiated histology in hepatoblastoma unfavorable? Hum Pathol 46 (4): 620-4, 2015.
- Vokuhl C, Oyen F, Häberle B, et al.: Small cell undifferentiated (SCUD) hepatoblastomas: All malignant rhabdoid tumors? Genes Chromosomes Cancer 55 (12): 925-931, 2016.
- Cornet M, De Lambert G, Pariente D, et al.: Rhabdoid tumor of the liver: Report of 6 pediatric cases treated at a single institute. J Pediatr Surg 53 (3): 567-571, 2018.
- Meyers RL, Tiao G, de Ville de Goyet J, et al.: Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 26 (1): 29-36, 2014.
- Malogolowkin MH, Katzenstein H, Krailo MD, et al.: Intensified platinum therapy is an ineffective strategy for improving outcome in pediatric patients with advanced hepatoblastoma. J Clin Oncol 24 (18): 2879-84, 2006.
- Katzenstein HM, Langham MR, Malogolowkin MH, et al.: Minimal adjuvant chemotherapy for children with hepatoblastoma resected at diagnosis (AHEP0731): a Children's Oncology Group, multicentre, phase 3 trial. Lancet Oncol 20 (5): 719-727, 2019.
- Hiyama E, Hishiki T, Watanabe K, et al.: Outcome and Late Complications of Hepatoblastomas Treated Using the Japanese Study Group for Pediatric Liver Tumor 2 Protocol. J Clin Oncol 38 (22): 2488-2498, 2020.
- Aronson DC, Weeda VB, Maibach R, et al.: Microscopically positive resection margin after hepatoblastoma resection: what is the impact on prognosis? A Childhood Liver Tumours Strategy Group (SIOPEL) report. Eur J Cancer 106: 126-132, 2019.
- Vasudevan SA, Meyers RL, Finegold MJ, et al.: Outcomes of children with well-differentiated fetal hepatoblastoma treated with surgery only: Report from Children's Oncology Group Trial, AHEP0731. J Pediatr Surg 57 (10): 251-256, 2022.
- Perilongo G, Maibach R, Shafford E, et al.: Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med 361 (17): 1662-70, 2009.
- Reyes JD, Carr B, Dvorchik I, et al.: Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr 136 (6): 795-804, 2000.
- Molmenti EP, Wilkinson K, Molmenti H, et al.: Treatment of unresectable hepatoblastoma with liver transplantation in the pediatric population. Am J Transplant 2 (6): 535-8, 2002.
- Czauderna P, Otte JB, Aronson DC, et al.: Guidelines for surgical treatment of hepatoblastoma in the modern era--recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL). Eur J Cancer 41 (7): 1031-6, 2005.
- Austin MT, Leys CM, Feurer ID, et al.: Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 41 (1): 182-6, 2006.
- Pham TA, Gallo AM, Concepcion W, et al.: Effect of Liver Transplant on Long-term Disease-Free Survival in Children With Hepatoblastoma and Hepatocellular Cancer. JAMA Surg 150 (12): 1150-8, 2015.
- Khan AS, Brecklin B, Vachharajani N, et al.: Liver Transplantation for Malignant Primary Pediatric Hepatic Tumors. J Am Coll Surg 225 (1): 103-113, 2017.
- Xianliang H, Jianhong L, Xuewu J, et al.: Cure of hepatoblastoma with transcatheter arterial chemoembolization. J Pediatr Hematol Oncol 26 (1): 60-3, 2004.
- Malogolowkin MH, Stanley P, Steele DA, et al.: Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol 18 (6): 1279-84, 2000.
- Aguado A, Dunn SP, Averill LW, et al.: Successful use of transarterial radioembolization with yttrium-90 (TARE-Y90) in two children with hepatoblastoma. Pediatr Blood Cancer 67 (9): e28421, 2020.
- Madanur MA, Battula N, Davenport M, et al.: Staged resection for a ruptured hepatoblastoma: a 6-year follow-up. Pediatr Surg Int 23 (6): 609-11, 2007.
- Aronson DC, Meyers RL: Malignant tumors of the liver in children. Semin Pediatr Surg 25 (5): 265-275, 2016.
- Venkatramani R, Stein JE, Sapra A, et al.: Effect of neoadjuvant chemotherapy on resectability of stage III and IV hepatoblastoma. Br J Surg 102 (1): 108-13, 2015.
- von Schweinitz D, Hecker H, Harms D, et al.: Complete resection before development of drug resistance is essential for survival from advanced hepatoblastoma--a report from the German Cooperative Pediatric Liver Tumor Study HB-89. J Pediatr Surg 30 (6): 845-52, 1995.
- Fuchs J, Cavdar S, Blumenstock G, et al.: POST-TEXT III and IV Hepatoblastoma: Extended Hepatic Resection Avoids Liver Transplantation in Selected Cases. Ann Surg 266 (2): 318-323, 2017.
- Hemming AW, Reed AI, Fujita S, et al.: Role for extending hepatic resection using an aggressive approach to liver surgery. J Am Coll Surg 206 (5): 870-5; discussion 875-8, 2008.
- Fonseca A, Gupta A, Shaikh F, et al.: Extreme hepatic resections for the treatment of advanced hepatoblastoma: Are planned close margins an acceptable approach? Pediatr Blood Cancer 65 (2): , 2018.
- de Freitas Paganoti G, Tannuri ACA, Dantas Marques AC, et al.: Extensive Hepatectomy as an Alternative to Liver Transplant in Advanced Hepatoblastoma: A New Protocol Used in a Pediatric Liver Transplantation Center. Transplant Proc 51 (5): 1605-1610, 2019.
- Katzenstein HM, Malogolowkin MH, Krailo MD, et al.: Doxorubicin in combination with cisplatin, 5-flourouracil, and vincristine is feasible and effective in unresectable hepatoblastoma: A Children's Oncology Group study. Cancer 128 (5): 1057-1065, 2022.
- Wang S, Yang C, Zhang J, et al.: First experience of high-intensity focused ultrasound combined with transcatheter arterial embolization as local control for hepatoblastoma. Hepatology 59 (1): 170-7, 2014.
- Meyers RL, Katzenstein HM, Krailo M, et al.: Surgical resection of pulmonary metastatic lesions in children with hepatoblastoma. J Pediatr Surg 42 (12): 2050-6, 2007.
- Karski EE, Dvorak CC, Leung W, et al.: Treatment of hepatoblastoma with high-dose chemotherapy and stem cell rescue: the pediatric blood and marrow transplant consortium experience and review of the literature. J Pediatr Hematol Oncol 36 (5): 362-8, 2014.
- Partrick DA, Bensard DD, Teitelbaum DH, et al.: Successful thoracoscopic lung biopsy in children utilizing preoperative CT-guided localization. J Pediatr Surg 37 (7): 970-3; discussion 970-3, 2002.
- Katzenstein HM, Rigsby C, Shaw PH, et al.: Novel therapeutic approaches in the treatment of children with hepatoblastoma. J Pediatr Hematol Oncol 24 (9): 751-5, 2002.
- Palmer RD, Williams DM: Dramatic response of multiply relapsed hepatoblastoma to irinotecan (CPT-11). Med Pediatr Oncol 41 (1): 78-80, 2003.
- Qayed M, Powell C, Morgan ER, et al.: Irinotecan as maintenance therapy in high-risk hepatoblastoma. Pediatr Blood Cancer 54 (5): 761-3, 2010.
- Zsíros J, Brugières L, Brock P, et al.: Efficacy of irinotecan single drug treatment in children with refractory or recurrent hepatoblastoma--a phase II trial of the childhood liver tumour strategy group (SIOPEL). Eur J Cancer 48 (18): 3456-64, 2012.
- Sue K, Ikeda K, Nakagawara A, et al.: Intrahepatic arterial injections of cisplatin-phosphatidylcholine-Lipiodol suspension in two unresectable hepatoblastoma cases. Med Pediatr Oncol 17 (6): 496-500, 1989.
- Habrand JL, Nehme D, Kalifa C, et al.: Is there a place for radiation therapy in the management of hepatoblastomas and hepatocellular carcinomas in children? Int J Radiat Oncol Biol Phys 23 (3): 525-31, 1992.
- Semeraro M, Branchereau S, Maibach R, et al.: Relapses in hepatoblastoma patients: clinical characteristics and outcome--experience of the International Childhood Liver Tumour Strategy Group (SIOPEL). Eur J Cancer 49 (4): 915-22, 2013.
- Shi Y, Geller JI, Ma IT, et al.: Relapsed hepatoblastoma confined to the lung is effectively treated with pulmonary metastasectomy. J Pediatr Surg 51 (4): 525-9, 2016.
- Matsunaga T, Sasaki F, Ohira M, et al.: Analysis of treatment outcome for children with recurrent or metastatic hepatoblastoma. Pediatr Surg Int 19 (3): 142-6, 2003.
- Malogolowkin MH, Katzenstein HM, Krailo M, et al.: Redefining the role of doxorubicin for the treatment of children with hepatoblastoma. J Clin Oncol 26 (14): 2379-83, 2008.
- Trobaugh-Lotrario AD, Meyers RL, Feusner JH: Outcomes of Patients With Relapsed Hepatoblastoma Enrolled on Children's Oncology Group (COG) Phase I and II Studies. J Pediatr Hematol Oncol 38 (3): 187-90, 2016.
- Yevich S, Calandri M, Gravel G, et al.: Reiterative Radiofrequency Ablation in the Management of Pediatric Patients with Hepatoblastoma Metastases to the Lung, Liver, or Bone. Cardiovasc Intervent Radiol 42 (1): 41-47, 2019.
- Liu B, Zhou L, Huang G, et al.: First Experience of Ultrasound-guided Percutaneous Ablation for Recurrent Hepatoblastoma after Liver Resection in Children. Sci Rep 5: 16805, 2015.
Hepatocellular Carcinoma
Incidence
The annual incidence of hepatocellular carcinoma in the United States is 0.4 cases per 1 million children between the ages of 0 and 14 years and 1.5 cases per 1 million adolescents aged 15 to 19 years.[
Fibrolamellar carcinoma of the liver was thought to be a subtype of hepatocellular carcinoma. However, it is now recognized as a distinct cancer. For more information, see the
Risk Factors
Conditions associated with hepatocellular carcinoma are described in
| Associated Disorder | Clinical Findings |
|---|---|
| Alagille syndrome[ |
Broad prominent forehead, deep-set eyes, and small prominent chin. Abnormality of bile ducts leads to intrahepatic scarring. For more information, see the |
| Glycogen storage diseases I–IV[ |
Symptoms vary by individual disorder. |
| Hepatitis B and C[ |
For more information, see the |
| Progressive familial intrahepatic cholestasis[ |
Symptoms of jaundice, pruritus, and failure to thrive begin in infancy and progress to portal hypertension and liver failure. |
| Tyrosinemia[ |
First few months of life: failure to thrive, vomiting, jaundice. |
Alagille syndrome
Alagille syndrome is an autosomal dominant genetic syndrome that is usually caused by a variant in or deletion of the JAG1 gene. It involves the bile ducts of the liver, the heart, and blood vessels in the brain and kidney. Patients develop a characteristic facies.[
Hepatitis B and hepatitis C infection
In children, hepatocellular carcinoma is associated with perinatally acquired HBV. In adults, it is associated with chronic HBV and hepatitis C virus (HCV) infection.[
HCV infection is associated with development of cirrhosis and hepatocellular carcinoma that takes decades to develop and is generally not seen in children.[
Nonviral liver injury
Specific types of nonviral liver injury and cirrhosis that are associated with hepatocellular carcinoma in children include the following:
- Tyrosinemia. Patients with tyrosinemia are regularly screened for hepatocellular carcinoma, even if they are treated with nitisinone.[
11 ] Nitisinone can prevent cirrhosis and decrease the incidence of hepatocellular carcinoma, especially when administered during infancy, after neonatal screening is used to diagnose tyrosinemia.[13 ] As of 2014, only a minority of state screening programs had adopted a highly recommended, new, more predictive newborn screen that is much more effective in newborn children aged 24 to 48 hours.[14 ]In an Iranian study, 36 children underwent liver transplant for tyrosinemia.[
15 ] Twenty-two children had liver nodules greater than 10 cm, and in 20 children, the nodules were cirrhotic. Median age at transplant was 3.9 years. Five of 19 children older than 2 years had hepatocellular carcinoma, and no children younger than 2 years had hepatocellular carcinoma in the resected liver. - Aggressive familial intrahepatic cholestasis. Hepatocellular carcinoma may also arise in very young children with variants in the ABCB11 gene (encodes bile salt export pump protein), which causes progressive familial intrahepatic cholestasis.[
9 ]
Genomics of Hepatocellular Carcinoma
Molecular features of hepatocellular carcinoma
Genomic findings related to hepatocellular carcinoma include the following:
- One case of pediatric hepatocellular carcinoma was analyzed by whole-exome sequencing, which showed a higher variant rate (53 variants) and the coexistence of CTNNB1 and NFE2L2 variants.[
16 ] - One study investigated pediatric (nonfibrolamellar) hepatocellular carcinoma tumors (N = 15) using multiple analytic tools. These tumors were found to frequently carry aberrations in a subset of genes that are commonly altered in adult hepatocellular carcinoma, including CTNNB1 and TERT. However, the molecular mechanisms of the variants are different. The TP53 variant was rare in this pediatric hepatocellular carcinoma cohort. Pediatric hepatocellular carcinoma that arose in the background of underlying metabolic disease had fewer variants and a distinct molecular profile. Typical driver variants were lacking in this group of patients.[
17 ] - A rare, more aggressive subtype of childhood liver cancer (hepatocellular neoplasm, not otherwise specified, also termed transitional liver cell tumor) occurs in older children. It has clinical and histopathological findings of both hepatoblastoma and hepatocellular carcinoma.
TERT variants were observed in two of four transitional liver cell tumor cases tested.[
18 ]TERT variants are also commonly observed in adults with hepatocellular carcinoma.[19 ]
To date, these genetic variants have not been used to select therapeutic agents for investigation in clinical trials.
Diagnosis
For more information, see the
Prognosis and Prognostic Factors
Prognosis
In the United states, the 5-year relative survival rate is 55% for children and adolescents with hepatocellular carcinoma.[
The 5-year OS rates by PRE-Treatment EXTent of disease (PRETEXT) group for patients with hepatocellular carcinoma in the SIOPEL-1 trial were found to be the following:[
- 44% for patients with PRETEXT I group tumors.
- 44% for patients with PRETEXT II group tumors.
- 22% for patients with PRETEXT III group tumors.
- 8% for patients with PRETEXT IV group tumors.
For more information about PRETEXT grouping, see the
Hepatocellular carcinoma prognosis by Evans surgical stage. Several staging systems exist for hepatocellular carcinoma, including the American Joint Committee on Cancer (AJCC) tumor-node-metastasis staging system (TNM) and the Barcelona Clinic Liver Cancer Staging System. However, the international prospective collaborative Paediatric Hepatic International Tumour Trial (PHITT) used the Evans Surgical Staging for childhood liver cancer. For more information, see the
- Stage I.
Children with stage I hepatocellular carcinoma have a good outcome.[
24 ] - Stage II.
Stage II is too rarely seen to predict outcome.
- Stages III and IV.
Stages III and IV are usually fatal.[
21 ,23 ]
Prognostic factors
Factors affecting prognosis include the following:
- Treatment-related factors: Cure of hepatocellular carcinoma requires gross tumor resection. However, hepatocellular carcinoma is often extensively invasive or multicentric, and less than 30% of tumors are resectable. Orthotopic liver transplant has been successful in selected children with hepatocellular carcinoma.[
25 ,26 ] - PRETEXT group: PRETEXT group (resectability) is also a prognostic factor. For more information, see the
Tumor Stratification by Imaging section. - Tumor histology: For more information, see the
Histology section.
Histology
The cells of hepatocellular carcinoma are epithelial in appearance. Hepatocellular carcinoma commonly arises in the right lobe of the liver.
Hepatocellular neoplasm, not otherwise specified (NOS)
Hepatocellular neoplasm, NOS, is also known as transitional liver cell tumor. This tumor, with characteristics of both hepatoblastoma and hepatocellular carcinoma, is a rare neoplasm found in older children and adolescents. It has a putative intermediate position between hepatoblasts and more mature hepatocyte-like tumor cells. The tumor cells may vary in regions of the tumor between classical hepatoblastoma and obvious hepatocellular carcinoma. In the international consensus classification, these tumors are referred to as hepatocellular neoplasm, NOS.[
Treatment of Hepatocellular Carcinoma
Treatment options for newly diagnosed hepatocellular carcinoma depend on the following:
- Whether the cancer is resectable at diagnosis.
- How the cancer responds to chemotherapy.
- Whether the cancer has metastasized.
- Whether the cancer is HBV related.
Treatment options for hepatocellular carcinoma that is resectable at diagnosis
Treatment options for hepatocellular carcinoma that is resectable at diagnosis include the following:
- Complete surgical resection of the primary tumor followed by chemotherapy.
- Chemotherapy followed by complete surgical resection of the primary tumor.[
23 ] - Complete surgical resection without chemotherapy.
Surgical resection and chemotherapy are the mainstays of treatment for resectable hepatocellular carcinoma.
Evidence (complete surgical resection followed by chemotherapy):
- Seven of eight patients with stage I hepatocellular carcinoma who received adjuvant cisplatin-based chemotherapy survived disease free.[
21 ] - In a survey of childhood liver tumors treated before the consistent use of chemotherapy, only 12 of 33 patients with hepatocellular carcinoma who had complete excision of the tumor survived.[
29 ] This suggests that treatment with adjuvant chemotherapy may benefit children with completely resected hepatocellular carcinoma. - In an analysis of SEER data for children and adolescents younger than 20 years who were diagnosed between 1976 and 2009, patients who underwent a complete resection had a 5-year OS rate of 60%, and patients who did not have a complete resection had a 5-year OS rate of 0%.[
22 ][Level of evidence C1]
Cisplatin and doxorubicin may be administered as adjuvant therapy because these agents may have activity in the treatment of hepatocellular carcinoma.[
Evidence (complete surgical resection without chemotherapy):
- In a single-institution retrospective report, 12 patients with stage I hepatocellular carcinoma were treated with surgery. Ten patients received no chemotherapy and two patients received a short course of chemotherapy based on oncologist preference.[
30 ][Level of evidence C1]- All 12 patients were alive without evidence of disease at a median of 54 months.
Despite improvements in surgical techniques, chemotherapy delivery, and patient supportive care in the past 20 years, clinical trials of chemotherapy have not shown improved survival rates for pediatric patients with hepatocellular carcinoma.[
Treatment options for nonmetastatic hepatocellular carcinoma that is not resectable at diagnosis
Treatment options for nonmetastatic hepatocellular carcinoma that is not resectable at diagnosis include the following:
- Chemotherapy followed by reassessment of surgical resectability. If the primary tumor is resectable, complete surgical resection.
- Chemotherapy with or without transarterial radioembolization (TARE) followed by reassessment of surgical resectability. If the primary tumor remains unresectable:
- Orthotopic liver transplant.
- Temporizing transarterial chemoembolization (TACE) or TARE followed by complete resection or liver transplant.
- TACE or TARE alone.
The use of neoadjuvant chemotherapy or TACE to enhance resectability or liver transplant, which may result in complete resection of tumor, is necessary for a cure.
Evidence (chemotherapy followed by surgery):
- In a prospective study of 41 patients who received preoperative cisplatin/doxorubicin chemotherapy, the following was observed:[
23 ]- Treatment resulted in a decrease in tumor size, with a decrease in alpha-fetoprotein (AFP) levels in about 50% of patients.
- The patients who responded to chemotherapy had a superior tumor resectability and survival rate. However, the OS rate was 28%, and only those who underwent complete resection survived.
Evidence (chemotherapy, TARE, or TACE followed by reassessment of surgical resectability; treatment options, including liver transplant, for unresectable primary tumor after chemotherapy, TARE, or TACE):
- Liver transplant has been a successful therapy for children with unresectable hepatocellular carcinoma. The survival rate is about 60%, with most deaths resulting from tumor recurrence.[
25 ,35 ,36 ,37 ,38 ] - A review of SEER data for hepatocellular carcinoma treatment in patients younger than 20 years revealed that 75% of patients underwent resection and 25% underwent liver transplant.[
39 ]- The 5-year OS rate was 53.4% with resection and 85.3% with transplant, suggesting that the criteria for transplant in hepatocellular carcinoma might be liberalized for overall patient benefit. This data has not been verified in a prospective clinical trial.
- TACE followed by complete surgical resection of the primary tumor may be an alternative to the use of chemotherapy followed by surgical resection.
- Studies in adults in China suggest that repeated hepatic TACE before surgery may improve the outcome of subsequent hepatectomy.[
40 ] - A meta-analysis found seven randomized trials that compared resection alone with TACE followed by resection. There was no difference in the 3-year event-free survival (EFS) and OS rates between the two groups, but the 5-year EFS and OS rates favored TACE followed by resection.[
41 ]
- Studies in adults in China suggest that repeated hepatic TACE before surgery may improve the outcome of subsequent hepatectomy.[
- TARE has been used in the treatment of adult patients with hepatocellular carcinoma for some time. In a small number of patients, TARE has provided both a palliative benefit and a possible bridge to liver transplant.[
42 ,43 ]
If the primary tumor is not resectable after chemotherapy and the patient is not a transplant candidate, alternative treatment approaches used in adults include the following:
- Sorafenib.
- TACE or TARE.
- Cryosurgery.
- Intratumoral injection of alcohol.
- Radiation therapy.
There are limited data on the use of these alternative treatment approaches in children.
Limited data from a European pilot study suggest that sorafenib was well tolerated in 12 children and adolescents with newly diagnosed advanced hepatocellular carcinoma when given in combination with standard chemotherapy of cisplatin and doxorubicin.[
Cryosurgery, intratumoral injection of alcohol, and radiofrequency ablation can successfully treat small (<5 cm) tumors in adults with cirrhotic livers.[
Most of the information about the use of targeted therapy or immunotherapy for patients with nonresectable hepatocellular carcinoma or metastatic disease has been informed by trials in adults. To learn more about these treatments in adults, see the
Treatment options for hepatocellular carcinoma with metastases at diagnosis
No specific treatment has proven effective for metastatic hepatocellular carcinoma in children and adolescents.
In two prospective trials, cisplatin plus either vincristine/fluorouracil or continuous-infusion doxorubicin was ineffective in adequately treating 25 patients with metastatic hepatocellular carcinoma.[
Treatment options for HBV-related hepatocellular carcinoma
Although HBV-related hepatocellular carcinoma is not common in children in the United States, nucleotide/nucleoside analog HBV inhibitor treatment improves postoperative prognosis in children and adults treated in China.[
Treatment options for HBV-related hepatocellular carcinoma include the following:
- Antiviral therapy.
Evidence (antiviral therapy):
- In a randomized controlled trial, 163 patients post–radical hepatectomy were evaluated for response to one of three antiviral treatments.[
49 ]- Antiviral treatment significantly decreased hepatocellular carcinoma recurrence, with a hazard ratio (HR) of 0.48 (95% confidence interval [CI], 0.32–0.70), and hepatocellular carcinoma–related death, with an HR of 0.26 (95% CI, 0.14–0.50), in multivariate Cox analyses.
- Patients who received antiviral treatment had significantly decreased early recurrence (HR, 0.41; 95% CI, 0.27–0.62) and improved liver function 6 months after surgery than did the control patients (P < .001).
Treatment of Progressive or Recurrent Hepatocellular Carcinoma
The prognosis for a patient with recurrent or progressive hepatocellular carcinoma is extremely poor.[
Treatment options for progressive or recurrent hepatocellular carcinoma include the following:
- Chemoembolization temporization before transplant or immediate liver transplant, for those with isolated recurrence in the liver.[
25 ,35 ,36 ,51 ] - Radiofrequency ablation.
In a retrospective single-institution study, ten children aged 6 to 17 years with recurrent hepatocellular carcinoma were treated with radiofrequency ablation. After one ablation, 14 of 15 target lesions had complete responses. None of these lesions progressed. The 1-year OS rate was 77.8%, and the 3-year OS rate was 44.4%.[
52 ][Level of evidence C1] - Phase I and phase II clinical trials may be appropriate and should be considered.
Treatment with sorafenib has resulted in improved progression-free survival in adults with advanced hepatocellular carcinoma. For adult patients who received sorafenib, the median survival and time to radiological progression were about 3 months longer than for patients who received a placebo.[
Treatment Options Under Clinical Evaluation for Hepatocellular Carcinoma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the
References:
- National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute.
Available online . Last accessed February 25, 2025. - El-Serag HB, Davila JA, Petersen NJ, et al.: The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 139 (10): 817-23, 2003.
- Chang MH, Chen TH, Hsu HM, et al.: Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res 11 (21): 7953-7, 2005.
- Keeffe EB, Pinson CW, Ragsdale J, et al.: Hepatocellular carcinoma in arteriohepatic dysplasia. Am J Gastroenterol 88 (9): 1446-9, 1993.
- Siciliano M, De Candia E, Ballarin S, et al.: Hepatocellular carcinoma complicating liver cirrhosis in type IIIa glycogen storage disease. J Clin Gastroenterol 31 (1): 80-2, 2000.
- Ni YH, Chang MH, Hsu HY, et al.: Hepatocellular carcinoma in childhood. Clinical manifestations and prognosis. Cancer 68 (8): 1737-41, 1991.
- Tsukuma H, Hiyama T, Tanaka S, et al.: Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 328 (25): 1797-801, 1993.
- González-Peralta RP, Langham MR, Andres JM, et al.: Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr 48 (5): 630-5, 2009.
- Knisely AS, Strautnieks SS, Meier Y, et al.: Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44 (2): 478-86, 2006.
- Alonso EM, Snover DC, Montag A, et al.: Histologic pathology of the liver in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr 18 (2): 128-33, 1994.
- van Spronsen FJ, Bijleveld CM, van Maldegem BT, et al.: Hepatocellular carcinoma in hereditary tyrosinemia type I despite 2-(2 nitro-4-3 trifluoro- methylbenzoyl)-1, 3-cyclohexanedione treatment. J Pediatr Gastroenterol Nutr 40 (1): 90-3, 2005.
- Park WS, Dong SM, Kim SY, et al.: Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res 59 (2): 307-10, 1999.
- de Laet C, Dionisi-Vici C, Leonard JV, et al.: Recommendations for the management of tyrosinaemia type 1. Orphanet J Rare Dis 8: 8, 2013.
- De Jesús VR, Adam BW, Mandel D, et al.: Succinylacetone as primary marker to detect tyrosinemia type I in newborns and its measurement by newborn screening programs. Mol Genet Metab 113 (1-2): 67-75, 2014 Sep-Oct.
- Bahador A, Dehghani SM, Geramizadeh B, et al.: Liver Transplant for Children With Hepatocellular Carcinoma and Hereditary Tyrosinemia Type 1. Exp Clin Transplant 13 (4): 329-32, 2015.
- Vilarinho S, Erson-Omay EZ, Harmanci AS, et al.: Paediatric hepatocellular carcinoma due to somatic CTNNB1 and NFE2L2 mutations in the setting of inherited bi-allelic ABCB11 mutations. J Hepatol 61 (5): 1178-83, 2014.
- Haines K, Sarabia SF, Alvarez KR, et al.: Characterization of pediatric hepatocellular carcinoma reveals genomic heterogeneity and diverse signaling pathway activation. Pediatr Blood Cancer 66 (7): e27745, 2019.
- Eichenmüller M, Trippel F, Kreuder M, et al.: The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J Hepatol 61 (6): 1312-20, 2014.
- Nault JC, Mallet M, Pilati C, et al.: High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun 4: 2218, 2013.
- Childhood cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). National Cancer Institute, 2012, Section 29.
Also available online . Last accessed August 11, 2022. - Katzenstein HM, Krailo MD, Malogolowkin MH, et al.: Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children's Cancer Group intergroup study. J Clin Oncol 20 (12): 2789-97, 2002.
- Allan BJ, Wang B, Davis JS, et al.: A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg 49 (1): 166-71; discussion 171, 2014.
- Czauderna P, Mackinlay G, Perilongo G, et al.: Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol 20 (12): 2798-804, 2002.
- Douglass E, Ortega J, Feusner J, et al.: Hepatocellular carcinoma (HCA) in children and adolescents: results from the Pediatric Intergroup Hepatoma Study (CCG 8881/POG 8945). [Abstract] Proceedings of the American Society of Clinical Oncology 13: A-1439, 420, 1994.
- Austin MT, Leys CM, Feurer ID, et al.: Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 41 (1): 182-6, 2006.
- Vinayak R, Cruz RJ, Ranganathan S, et al.: Pediatric liver transplantation for hepatocellular cancer and rare liver malignancies: US multicenter and single-center experience (1981-2015). Liver Transpl 23 (12): 1577-1588, 2017.
- López-Terrada D, Alaggio R, de Dávila MT, et al.: Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 27 (3): 472-91, 2014.
- Prokurat A, Kluge P, Kościesza A, et al.: Transitional liver cell tumors (TLCT) in older children and adolescents: a novel group of aggressive hepatic tumors expressing beta-catenin. Med Pediatr Oncol 39 (5): 510-8, 2002.
- Exelby PR, Filler RM, Grosfeld JL: Liver tumors in children in the particular reference to hepatoblastoma and hepatocellular carcinoma: American Academy of Pediatrics Surgical Section Survey--1974. J Pediatr Surg 10 (3): 329-37, 1975.
- D'Souza AM, Shah R, Gupta A, et al.: Surgical management of children and adolescents with upfront completely resected hepatocellular carcinoma. Pediatr Blood Cancer 65 (11): e27293, 2018.
- Murawski M, Weeda VB, Maibach R, et al.: Hepatocellular Carcinoma in Children: Does Modified Platinum- and Doxorubicin-Based Chemotherapy Increase Tumor Resectability and Change Outcome? Lessons Learned From the SIOPEL 2 and 3 Studies. J Clin Oncol 34 (10): 1050-6, 2016.
- Kelly D, Sharif K, Brown RM, et al.: Hepatocellular carcinoma in children. Clin Liver Dis 19 (2): 433-47, 2015.
- Malek MM, Shah SR, Atri P, et al.: Review of outcomes of primary liver cancers in children: our institutional experience with resection and transplantation. Surgery 148 (4): 778-82; discussion 782-4, 2010.
- Ismail H, Broniszczak D, Kaliciński P, et al.: Liver transplantation in children with hepatocellular carcinoma. Do Milan criteria apply to pediatric patients? Pediatr Transplant 13 (6): 682-92, 2009.
- Pham TA, Gallo AM, Concepcion W, et al.: Effect of Liver Transplant on Long-term Disease-Free Survival in Children With Hepatoblastoma and Hepatocellular Cancer. JAMA Surg 150 (12): 1150-8, 2015.
- Reyes JD, Carr B, Dvorchik I, et al.: Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr 136 (6): 795-804, 2000.
- Bilik R, Superina R: Transplantation for unresectable liver tumors in children. Transplant Proc 29 (7): 2834-5, 1997.
- Romano F, Stroppa P, Bravi M, et al.: Favorable outcome of primary liver transplantation in children with cirrhosis and hepatocellular carcinoma. Pediatr Transplant 15 (6): 573-9, 2011.
- McAteer JP, Goldin AB, Healey PJ, et al.: Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatr Transplant 17 (8): 744-50, 2013.
- Zhang Z, Liu Q, He J, et al.: The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma. Cancer 89 (12): 2606-12, 2000.
- Yu T, Xu X, Chen B: TACE combined with liver resection versus liver resection alone in the treatment of resectable HCC: a meta-analysis. Chinese-German J Clin Oncol 12 (11): 532-6, 2013.
- Tohme S, Bou Samra P, Kaltenmeier C, et al.: Radioembolization for Hepatocellular Carcinoma: A Nationwide 10-Year Experience. J Vasc Interv Radiol 29 (7): 912-919.e2, 2018.
- Aguado A, Ristagno R, Towbin AJ, et al.: Transarterial radioembolization with yttrium-90 of unresectable primary hepatic malignancy in children. Pediatr Blood Cancer 66 (7): e27510, 2019.
- Schmid I, Häberle B, Albert MH, et al.: Sorafenib and cisplatin/doxorubicin (PLADO) in pediatric hepatocellular carcinoma. Pediatr Blood Cancer 58 (4): 539-44, 2012.
- Zhou XD, Tang ZY: Cryotherapy for primary liver cancer. Semin Surg Oncol 14 (2): 171-4, 1998.
- Lencioni RA, Allgaier HP, Cioni D, et al.: Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 228 (1): 235-40, 2003.
- Lubienski A: Hepatocellular carcinoma: interventional bridging to liver transplantation. Transplantation 80 (1 Suppl): S113-9, 2005.
- Weiss KE, Sze DY, Rangaswami AA, et al.: Transarterial chemoembolization in children to treat unresectable hepatocellular carcinoma. Pediatr Transplant 22 (4): e13187, 2018.
- Yin J, Li N, Han Y, et al.: Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol 31 (29): 3647-55, 2013.
- Malogolowkin MH, Stanley P, Steele DA, et al.: Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol 18 (6): 1279-84, 2000.
- Otte JB, Pritchard J, Aronson DC, et al.: Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer 42 (1): 74-83, 2004.
- Long H, Wu W, Zhou L, et al.: Radiofrequency ablation for pediatric recurrent hepatocellular carcinoma: a single-center experience. BMC Med Imaging 23 (1): 202, 2023.
- Llovet JM, Ricci S, Mazzaferro V, et al.: Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359 (4): 378-90, 2008.
Fibrolamellar Carcinoma
Fibrolamellar carcinoma was previously considered a rare subtype of hepatocellular carcinoma. It is also called fibrolamellar hepatocellular carcinoma and fibrolamellar liver cancer. With the 2014 discovery of a pathognomonic DNAJB::PRKACA chimera that defines this entity, it has been redefined as a distinct type of cancer, separate from hepatocellular carcinoma.[
Incidence
Fibrolamellar carcinoma most commonly arises in adolescents and adults, although it can also arise in young children and older adults.[
Risk Factors
Carney complex is caused by heterozygous germline pathogenic variants in PRKAR1A and is an autosomal dominant genetic syndrome.[
Diagnosis
Fibrolamellar carcinoma was first described as a distinct pathological entity by Edmonson in 1956. It is characterized by large cells with eosinophilic cytoplasm, central nuclei with vesiculated chromatin and prominent macronucleoli, along with dense bands of lamellar fibrosis that gives the tumor its name.[
Genomics of Fibrolamellar Carcinoma
Molecular features of fibrolamellar carcinoma
Fibrolamellar carcinoma is a rare subtype of hepatocellular carcinoma observed in older children and young adults. It is characterized by an approximately 400 kB deletion on chromosome 19, which produces a chimeric transcript. This chimeric RNA codes for a protein containing the amino-terminal domain of DNAJB1, a homolog of the molecular chaperone DNAJ, fused in frame with PRKACA, the catalytic domain of protein kinase A.[
Prognosis
Fibrolamellar carcinoma is not associated with cirrhosis of the liver. It was previously thought to confer a more favorable prognosis than hepatocellular carcinoma.[
Treatment of Fibrolamellar Carcinoma
Surgery is the standard of care for patients with radiographically localized fibrolamellar carcinoma. For patients with distant spread of disease, systemic therapy is based on the current treatments for pediatric or adult hepatocellular carcinoma, albeit with similarly poor effectiveness. For more information about the treatments used for fibrolamellar carcinoma, see the
Treatment Options Under Clinical Evaluation for Fibrolamellar Carcinoma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the
References:
- Honeyman JN, Simon EP, Robine N, et al.: Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 343 (6174): 1010-4, 2014.
- Cruz O, Laguna A, Vancells M, et al.: Fibrolamellar hepatocellular carcinoma in an infant and literature review. J Pediatr Hematol Oncol 30 (12): 968-71, 2008.
- Eggert T, McGlynn KA, Duffy A, et al.: Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: A detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J 1 (5): 351-7, 2013.
- Zack T, Losert KP, Maisel SM, et al.: Defining incidence and complications of fibrolamellar liver cancer through tiered computational analysis of clinical data. NPJ Precis Oncol 7 (1): 29, 2023.
- El-Serag HB, Davila JA, Petersen NJ, et al.: The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 139 (10): 817-23, 2003.
- Stratakis CA: Carney Complex. In: Adam MP, Feldman J, Mirzaa GM, et al., eds.: GeneReviews. University of Washington, Seattle, 1993-2024, pp.
Available online . Last accessed February 29, 2024. - Graham RP, Lackner C, Terracciano L, et al.: Fibrolamellar carcinoma in the Carney complex: PRKAR1A loss instead of the classic DNAJB1-PRKACA fusion. Hepatology 68 (4): 1441-1447, 2018.
- EDMONDSON HA: Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child 91 (2): 168-86, 1956.
- Mayo SC, Mavros MN, Nathan H, et al.: Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg 218 (2): 196-205, 2014.
- Czauderna P, Mackinlay G, Perilongo G, et al.: Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol 20 (12): 2798-804, 2002.
- Katzenstein HM, Krailo MD, Malogolowkin MH, et al.: Fibrolamellar hepatocellular carcinoma in children and adolescents. Cancer 97 (8): 2006-12, 2003.
- Weeda VB, Murawski M, McCabe AJ, et al.: Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma--results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer 49 (12): 2698-704, 2013.
Undifferentiated Embryonal Sarcoma of the Liver
Incidence
Undifferentiated embryonal sarcoma of the liver (UESL) is a distinct clinical and pathological entity and accounts for 2% to 15% of pediatric hepatic malignancies.[
Diagnosis
UESL presents as an abdominal mass, often with pain or malaise, usually between the ages of 5 and 10 years. Widespread infiltration throughout the liver and pulmonary metastasis are common. It may appear solid or cystic on imaging, frequently with central necrosis. Undifferentiated sarcomas, like small cell undifferentiated hepatoblastomas, should be examined for loss of SMARCB1 expression by immunohistochemistry to help rule out rhabdoid tumor of the liver.
It is important to make the diagnostic distinction between UESL and biliary tract rhabdomyosarcoma because they share some common clinical and pathological features, but treatment differs between the two, as shown in
| | Undifferentiated Embryonal Sarcoma of the Liver | Biliary Tract Rhabdomyosarcoma |
|---|---|---|
| a Adapted from Nicol et al.[ |
||
| Median Age at Diagnosis | 10.5 y | 3.4 y |
| Tumor Location | Often arises in the right lobe of the liver | Often arises in the hilum of the liver |
| Biliary Obstruction | Unusual | Frequent; jaundice is a common presenting symptom |
| Treatment | Surgery and chemotherapy | Surgery (usually biopsy only), radiation therapy, and chemotherapy |
Histology
Distinctive histological features of UESL include intracellular hyaline globules and marked anaplasia on a mesenchymal background.[
Strong clinical and histological evidence suggests that UESL can arise within preexisting mesenchymal hamartomas of the liver, which are large, benign, multicystic masses that present in the first 2 years of life.[
Prognosis and Prognostic Factors
The overall survival (OS) rates of children with UESL appear to be substantially higher than 50% when combining reports, although all series are small and may have selection bias.[
The Childhood Cancer Database, which does not provide central review of pathology or reliable details of nonsurgical treatment, reported on 103 children with UESL diagnosed between 1998 and 2012. The 5-year OS rate was 86% for all patients and 92% for those treated with combination surgery and chemotherapy. A multivariate analysis of the nonsurgical data revealed statistically significant poorer outcomes for patients with tumors larger than 15 cm. Seven of ten children who presented with metastases and ten of ten children who underwent orthotopic liver transplant survived at least 5 years, but details of their treatment were not presented.[
A retrospective study combined data from three European studies to identify 64 patients with UESL.[
Treatment Options for UESL
UESL is rare. Only small series have been published regarding treatment.[
Treatment options for UESL include the following:
- Surgical resection and chemotherapy.
- Liver transplant for unresectable tumors.
The generally accepted approach is resection of the primary tumor mass in the liver when possible.[
Evidence (surgical resection and chemotherapy):
- The Italian and German Soft Tissue Sarcoma Cooperative Groups prospectively studied patients with UESL. Patients were treated with conservative surgery or biopsy followed by neoadjuvant chemotherapy consisting of varying combinations of vincristine, cyclophosphamide, dactinomycin, doxorubicin, and ifosfamide. Disease evaluation, usually after four cycles of chemotherapy, was followed by second-look surgery when appropriate to try to remove residual primary tumor, followed by additional and/or adjuvant chemotherapy.[
12 ]- Ten of 17 patients survived in first complete remission, and one patient survived in third complete remission.
- In a subset analysis from the Children's Oncology Group ARST0332 (NCT00346164) study, 39 patients with embryonal sarcoma of the liver were available for analysis. Patients underwent upfront (n = 23) or delayed (n = 16) resection and received adjuvant or neoadjuvant chemotherapy (dose-intensive ifosfamide/doxorubicin). Eight patients received radiation therapy.[
22 ]- The 5-year EFS rate was 79% (95% CI, 65%–93%), and the 5-year OS rate was 95% (95% CI, 87%–100%).
- In a single-center retrospective report, five patients with UESL were treated with surgery and adjuvant chemotherapy consisting of vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide. Four patients had stage I disease, and one patient had stage II disease. One patient received abdominal radiation for tumor rupture.[
17 ][Level of evidence C1]- All patients were alive (range, 5–19 years), with EFS and OS rates of 100%.
Liver transplant has occasionally been used to successfully treat an otherwise unresectable primary tumor.[
Treatment Options Under Clinical Evaluation for UESL
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the
References:
- Nicol K, Savell V, Moore J, et al.: Distinguishing undifferentiated embryonal sarcoma of the liver from biliary tract rhabdomyosarcoma: a Children's Oncology Group study. Pediatr Dev Pathol 10 (2): 89-97, 2007 Mar-Apr.
- Stocker JT: Hepatic tumors in children. Clin Liver Dis 5 (1): 259-81, viii-ix, 2001.
- Shehata BM, Gupta NA, Katzenstein HM, et al.: Undifferentiated embryonal sarcoma of the liver is associated with mesenchymal hamartoma and multiple chromosomal abnormalities: a review of eleven cases. Pediatr Dev Pathol 14 (2): 111-6, 2011 Mar-Apr.
- Stringer MD, Alizai NK: Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg 40 (11): 1681-90, 2005.
- O'Sullivan MJ, Swanson PE, Knoll J, et al.: Undifferentiated embryonal sarcoma with unusual features arising within mesenchymal hamartoma of the liver: report of a case and review of the literature. Pediatr Dev Pathol 4 (5): 482-9, 2001 Sep-Oct.
- Walther A, Geller J, Coots A, et al.: Multimodal therapy including liver transplantation for hepatic undifferentiated embryonal sarcoma. Liver Transpl 20 (2): 191-9, 2014.
- Ismail H, Dembowska-Bagińska B, Broniszczak D, et al.: Treatment of undifferentiated embryonal sarcoma of the liver in children--single center experience. J Pediatr Surg 48 (11): 2202-6, 2013.
- Chowdhary SK, Trehan A, Das A, et al.: Undifferentiated embryonal sarcoma in children: beware of the solitary liver cyst. J Pediatr Surg 39 (1): E9-12, 2004.
- Baron PW, Majlessipour F, Bedros AA, et al.: Undifferentiated embryonal sarcoma of the liver successfully treated with chemotherapy and liver resection. J Gastrointest Surg 11 (1): 73-5, 2007.
- Kim DY, Kim KH, Jung SE, et al.: Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg 37 (10): 1419-23, 2002.
- Webber EM, Morrison KB, Pritchard SL, et al.: Undifferentiated embryonal sarcoma of the liver: results of clinical management in one center. J Pediatr Surg 34 (11): 1641-4, 1999.
- Bisogno G, Pilz T, Perilongo G, et al.: Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer 94 (1): 252-7, 2002.
- Urban CE, Mache CJ, Schwinger W, et al.: Undifferentiated (embryonal) sarcoma of the liver in childhood. Successful combined-modality therapy in four patients. Cancer 72 (8): 2511-6, 1993.
- Okajima H, Ohya Y, Lee KJ, et al.: Management of undifferentiated sarcoma of the liver including living donor liver transplantation as a backup procedure. J Pediatr Surg 44 (2): e33-8, 2009.
- Weitz J, Klimstra DS, Cymes K, et al.: Management of primary liver sarcomas. Cancer 109 (7): 1391-6, 2007.
- Plant AS, Busuttil RW, Rana A, et al.: A single-institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (UELS): success of combined therapy and the use of orthotopic liver transplant. J Pediatr Hematol Oncol 35 (6): 451-5, 2013.
- Mathias MD, Ambati SR, Chou AJ, et al.: A single-center experience with undifferentiated embryonal sarcoma of the liver. Pediatr Blood Cancer 63 (12): 2246-2248, 2016.
- Shi Y, Rojas Y, Zhang W, et al.: Characteristics and outcomes in children with undifferentiated embryonal sarcoma of the liver: A report from the National Cancer Database. Pediatr Blood Cancer 64 (4): , 2017.
- Guérin F, Martelli H, Rogers T, et al.: Outcome of patients with undifferentiated embryonal sarcoma of the liver treated according to European soft tissue sarcoma protocols. Pediatr Blood Cancer 70 (7): e30374, 2023.
- Techavichit P, Masand PM, Himes RW, et al.: Undifferentiated Embryonal Sarcoma of the Liver (UESL): A Single-Center Experience and Review of the Literature. J Pediatr Hematol Oncol 38 (4): 261-8, 2016.
- Merli L, Mussini C, Gabor F, et al.: Pitfalls in the surgical management of undifferentiated sarcoma of the liver and benefits of preoperative chemotherapy. Eur J Pediatr Surg 25 (1): 132-7, 2015.
- Spunt SL, Xue W, Gao Z, et al.: Embryonal sarcoma of the liver in pediatric and young adult patients: A report from Children's Oncology Group study ARST0332. Cancer 130 (15): 2683-2693, 2024.
- Kelly MJ, Martin L, Alonso M, et al.: Liver transplant for relapsed undifferentiated embryonal sarcoma in a young child. J Pediatr Surg 44 (12): e1-3, 2009.
Infantile Choriocarcinoma of the Liver
Choriocarcinoma of the liver is a very rare tumor that appears to originate in the placenta during gestation. It presents with a liver mass in the first few months of life. Metastasis from the placenta to maternal tissues occurs in many cases, necessitating beta-human chorionic gonadotropin (beta-hCG) testing of the mother. Infants are often unstable at diagnosis because of hemorrhage of the tumor.
Diagnosis
Clinical diagnosis may be made without biopsy on the basis of tumor imaging of the liver associated with extremely high serum beta-hCG levels and alpha-fetoprotein (AFP) levels in the reference range for age.[
Histology
Cytotrophoblasts and syncytiotrophoblasts are both present. The former are closely packed nests of medium-sized cells with clear cytoplasm, distinct cell margins, and vesicular nuclei. The latter are very large, multinucleated syncytia formed from the cytotrophoblasts.[
Prognosis
The prognosis of patients with infantile choriocarcinoma of the liver is often poor because of the instability at presentation from hemorrhage. A 2017 case report and literature review found 32 cases, with 6 long-term survivors. The authors emphasized the opportunity for early diagnosis and treatment of this very chemosensitive tumor.[
Treatment Options for Infantile Choriocarcinoma of the Liver
Treatment options for infantile choriocarcinoma of the liver include the following:
- Surgical resection.[
1 ] - Chemotherapy followed by surgical resection.
- Chemotherapy followed by liver transplant.
Initial surgical removal of the tumor mass may be difficult because of its friability and hemorrhagic tendency. Surgical removal of the primary tumor is often performed after neoadjuvant chemotherapy.[
Maternal gestational trophoblastic tumors are exquisitely sensitive to methotrexate. Many women, including those with distant metastases, are cured with single-agent chemotherapy. Maternal and infantile choriocarcinoma both come from the same placental malignancy. The combination of cisplatin, etoposide, and bleomycin, as used in other pediatric germ cell tumors, has been effective in some patients and is followed by resection of the residual mass. Use of neoadjuvant methotrexate in infantile choriocarcinoma, although often resulting in a response, has not been uniformly successful.[
A case report of neoadjuvant chemotherapy followed by successful liver transplant highlights the opportunity for this therapy in children whose tumors remain unresectable after chemotherapy.[
Treatment Options Under Clinical Evaluation for Infantile Choriocarcinoma of the Liver
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the
References:
- Yoon JM, Burns RC, Malogolowkin MH, et al.: Treatment of infantile choriocarcinoma of the liver. Pediatr Blood Cancer 49 (1): 99-102, 2007.
- Olson T, Schneider D, Perlman E: Germ cell tumors. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 6th ed. Lippincott Williams and Wilkins, 2011, pp 1045-1067.
- Alsharif S, Karsou A: Infantile choriocarcinoma of the liver: case report and review of the literature. Oncol Cancer Case Rep 3 (1): 2017.
- Hanson D, Walter AW, Dunn S, et al.: Infantile choriocarcinoma in a neonate with massive liver involvement cured with chemotherapy and liver transplant. J Pediatr Hematol Oncol 33 (6): e258-60, 2011.
Vascular Liver Tumors
Careful attention to clinical history, physical examination, laboratory evaluation, and radiological imaging is essential for an appropriate diagnosis of vascular liver tumors. If there is any doubt about the accuracy of the diagnosis, a biopsy should be performed.
The different diagnoses of vascular tumors of the liver include the following:
- Benign tumors.
- Focal congenital hemangiomas. For more information, see the
Congenital Hemangiomas section in Childhood Vascular Tumors Treatment. - Multiple or diffuse infantile hemangiomas. For more information, see the
Infantile Hemangioma section in Childhood Vascular Tumors Treatment.
- Focal congenital hemangiomas. For more information, see the
- Malignant tumors.
- Epithelioid hemangioendothelioma. For more information, see the
Epithelioid Hemangioendothelioma section in Childhood Vascular Tumors Treatment. - Angiosarcoma. For more information, see the
Angiosarcoma section in Childhood Vascular Tumors Treatment.
- Epithelioid hemangioendothelioma. For more information, see the
Current Clinical Trials
Use our
Latest Updates to This Summary (01 / 06 / 2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood liver cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Liver Cancer Treatment are:
- Denise Adams, MD (Children's Hospital Boston)
- Andrea A. Hayes-Dixon, MD, FACS, FAAP (Howard University)
- Karen J. Marcus, MD, FACR (Dana-Farber of Boston Children's Cancer Center and Blood Disorders Harvard Medical School)
- Thomas A. Olson, MD (Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta - Egleston Campus)
- Michael V. Ortiz, MD (Memorial Sloan Kettering Cancer Center)
- Stephen J. Shochat, MD (St. Jude Children's Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Liver Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at:
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our
Last Revised: 2025-01-06
This information does not replace the advice of a doctor. Ignite Healthwise, LLC, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.
Page Footer
I want to...
Audiences
Secure Member Sites
The Cigna Group Information
Disclaimer
Individual and family medical and dental insurance plans are insured by Cigna Health and Life Insurance Company (CHLIC), Cigna HealthCare of Arizona, Inc., Cigna HealthCare of Illinois, Inc., Cigna HealthCare of Georgia, Inc., Cigna HealthCare of North Carolina, Inc., Cigna HealthCare of South Carolina, Inc., and Cigna HealthCare of Texas, Inc. Group health insurance and health benefit plans are insured or administered by CHLIC, Connecticut General Life Insurance Company (CGLIC), or their affiliates (see
All insurance policies and group benefit plans contain exclusions and limitations. For availability, costs and complete details of coverage, contact a licensed agent or Cigna sales representative. This website is not intended for residents of New Mexico.