Treatment Option Overview
There are different types of treatment for children and adolescents with testicular cancer.
Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment.
Because cancer in children is rare, taking part in a clinical trial should be considered. Some clinical trials are open only to patients who have not started treatment.
Children and adolescents with testicular cancer should have their treatment planned by a team of doctors who are experts in treating childhood cancer.
Treatment will be overseen by a pediatric oncologist, a doctor who specializes in treating children with cancer. The pediatric oncologist works with other pediatric health professionals who are experts in treating children with cancer and who specialize in certain areas of medicine. This may include the following specialists and others:
- Pediatrician.
- Pediatric surgeon.
- Pediatric urologist.
- Pediatric nurse specialist.
- Rehabilitation specialist.
- Social worker.
One type of standard treatment is used for testicular cancer.
Surgery
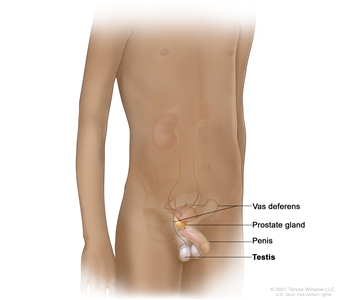
Surgery is used to remove the cancer from the testicle or to remove one or both testicles.
New types of treatment are being tested in clinical trials.
This summary section describes treatments that are being studied in clinical trials. It may not mention every new treatment being studied. Information about clinical trials is available from the NCI website.
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do.
Targeted therapy is being studied for the treatment of childhood testicular cancer that has recurred (come back).
Treatment for childhood testicular cancer may cause side effects.
For information about side effects that begin during treatment for cancer, see our Side Effects page.
Side effects from cancer treatment that begin after treatment and continue for months or years are called late effects. Physical problems, such as problems with fertility, are a late effect of treatment.
Some late effects may be treated or controlled. It is important to talk with your child's doctors about the possible late effects caused by some treatments. See the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI's clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
Some of the tests that were done to diagnose the cancer or to find out the stage of the cancer may be repeated. Some tests will be repeated in order to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your child's condition has changed or if the cancer has recurred (come back). These tests are sometimes called follow-up tests or check-ups.