Shop for Plans
Shop for your own coverage
Plans through your employer
Learn about the medical, dental, pharmacy, behavioral, and voluntary benefits your employer may offer.
Learn
Living or working abroad?
Genetics of Breast and Gynecologic Cancers (PDQ®): Genetics - Health Professional Information [NCI]
Executive Summary
This executive summary reviews the topics covered in this PDQ summary on the genetics of breast and gynecologic cancers.
- Inheritance and Risk
Factors suggestive of a
genetic contribution to both breast cancer and gynecologic cancer include 1) an increased incidence of these cancers among individuals with a family history of these cancers, 2) multiple family members affected with these and other cancers, and 3) a pattern of cancers compatible with autosomal dominant inheritance. Both males and females can inherit and transmit an autosomal dominant cancer predisposition gene.Additional factors coupled with family history can influence an individual's risk of developing cancer. These factors include
reproductive history ,contraceptive andhormone replacement use ,radiation exposure early in life,alcohol consumption and smoking , andphysical activity .Risk assessment models have been developed to clarify an individual's 1)
lifetime risk of developing breast and/or gynecologic cancer , 2)likelihood of having a pathogenic variant in BRCA1 or BRCA2 , and 3)likelihood of having a pathogenic variant in one of the mismatch repair genes associated with Lynch syndrome .
- Associated Genes and Syndromes
Breast and ovarian cancer are present in several autosomal dominant cancer syndromes, although they are most strongly associated with highly penetrant germline pathogenic variants in
BRCA1 and BRCA2 . Other genes, such asPALB2 ,TP53 (associated with Li-Fraumeni syndrome),PTEN (associated with PTEN hamartoma tumor syndromes, including Cowden syndrome),CDH1 (associated with diffuse gastric and lobular breast cancer syndrome), andSTK11 (associated with Peutz-Jeghers syndrome), confer a risk to either or both of these cancers with relatively high penetrance.Inherited endometrial cancer is most commonly associated with
Lynch syndrome , a condition caused by inherited pathogenic variants in the highly penetrant mismatch repair genes MLH1, MSH2, MSH6, PMS2, and EPCAM. Colorectal cancer (and, to a lesser extent, ovarian cancer and stomach cancer) is also associated with Lynch syndrome.CHEK2 ,BRIP1 ,RAD51C ,RAD51D , andATM are moderate penetrance genes that are associated with increased breast and/or gynecologic cancer risk.Genome-wide searches are showing promise in identifying common, low-penetrance susceptibility alleles for many complex diseases, including breast and gynecologic cancers, but the clinical utility of these findings remains uncertain.
- Clinical Management
Breast cancer screening strategies , including breast magnetic resonance imaging and mammography, are commonly performed in carriers of BRCA pathogenic variants and in individuals at increased risk of breast cancer. Initiation of screening is generally recommended at earlier ages and at more frequent intervals in individuals with an increased risk due to genetics and family history than in the general population. There is evidence to demonstrate that these strategies have utility in early detection of cancer. In contrast, there is currently no evidence to demonstrate thatovarian cancer screening using cancer antigen–125 testing and transvaginal ultrasound leads to early detection of cancer.Risk-reducing surgeries, including
risk-reducing mastectomy (RRM) andrisk-reducing salpingo-oophorectomy (RRSO), have been shown to significantly reduce the risk of developing breast and/or ovarian cancer and improve overall survival in carriers of BRCA1 and BRCA2 pathogenic variants.Chemoprevention strategies for breast cancer andchemoprevention strategies for ovarian cancer have been examined in this population. For example,tamoxifen use has been shown to reduce the risk ofcontralateral breast cancer among carriers of BRCA1 and BRCA2 pathogenic variants after treatment for breast cancer, but there are limited data in the primary cancer prevention setting to suggest that it reduces the risk of breast cancer among healthy female carriers of BRCA2 pathogenic variants. The use oforal contraceptives also has been associated with a protective effect on the risk of developing ovarian cancer, including in carriers of BRCA1 and BRCA2 pathogenic variants, with no association of increased risk of breast cancer when using formulations developed after 1975.
- Psychosocial and Behavioral Issues
Psychosocial factors influence decisions about genetic testing for inherited cancer risk and risk-management strategies.Uptake of genetic testing varies widely across studies. Psychological factors that have been associated with testing uptake include cancer-specific distress and perceived risk of developing breast or ovarian cancer. Studies have shownlow levels of distress after genetic testing for both carriers and noncarriers, particularly in the longer term.Uptake of RRM and RRSO also varies across studies and may be influenced by factors such as cancer history, age, family history, recommendations of the health care provider, and pretreatment genetic education and counseling.Patients' communication with their family members about an inherited risk of breast and gynecologic cancer is complex; gender, age, and the degree of relatedness are some elements that affect disclosure of this information. Research is ongoing to better understand and address psychosocial and behavioral issues in high-risk families.
Introduction
General Information
Among women in the United States, breast cancer is the most commonly diagnosed cancer after nonmelanoma skin cancer, and it is the second leading cause of cancer deaths after lung cancer. In 2025, an estimated 319,750 new cases of breast cancer (including 2,800 cases in men) will be diagnosed, and 42,680 deaths (including 510 deaths in men) will occur.[
A possible genetic contribution to both breast and ovarian cancer risk is indicated by the increased incidence of these cancers among women with a family history (refer to the
Risk Factors for Breast Cancer
This section discusses factors that can modify an individual's risk of developing breast cancer. These risk factors can affect women in the general population, women who have a family histories of breast cancer, and women who carry pathogenic variants in breast cancer risk genes. For more information on breast cancer risk factors in the general population, see
The following breast cancer risk factors are discussed in this section:
-
Age . -
Family history of breast cancer . -
Benign breast disease, mammographic density, and background parenchymal enhancement . -
Parity, age at first birth, and breastfeeding . -
Contraceptives . -
Hormone replacement therapy (HRT) . -
Radiation exposure . -
Alcohol and smoking . -
Physical activity .
These factors can increase or decrease breast cancer risk in all women. However, they may affect breast cancer risk differently in women with increased breast cancer susceptibility (i.e., women who have high-risk family histories and/or pathogenic variants in hereditary breast cancer genes). Factors that increase breast cancer risk in the general population may lower breast cancer risk, increase breast cancer risk more than expected, or have no effect on breast cancer risk in women with high breast cancer susceptibility. In some cases, these risk factors may affect high-risk women in the same way that they affect average-risk women. Furthermore, modifying risk factors has a greater effect on the absolute breast cancer risk in women with high breast cancer susceptibility than in women with low breast cancer susceptibility.[
Age
Like other cancer types, breast cancer's cumulative risk increases with age. As individuals age, they encounter more environmental exposures and accumulate genomic changes. Hence, most breast cancers occur after age 50 years.[
Family history of breast cancer
A family history of breast cancer is a well-established, consistent risk factor for breast cancer. Approximately 5% to 10% of women with breast cancer also had a mother or sister with breast cancer in cross-sectional studies. About 10% to 20% of women had a first-degree relative (FDR) or a second-degree relative (SDR) with breast cancer.[
The following factors can increase a woman's breast cancer risk:
- Large number of affected relatives.
- Family members who were diagnosed with breast cancer at young ages.
- Family members with bilateral breast cancers.
- Family members with multiple ipsilateral breast cancers.
- Male relatives with breast cancer.
Furthermore, women with family histories of multiple breast cancers had higher hazard ratios (HRs) (HR, 2.7; 95% CI, 2.6–2.9) than women who had a single breast cancer in their families (HR, 1.8; 95% CI, 1.8–1.9). When women had multiple breast cancers in their families (with one breast cancer occurring before age 40 years), the HR was 3.8 (95% CI, 3.1–4.8). However, breast cancer risk also significantly increased when a relative was diagnosed with breast cancer at 60 years or older, suggesting that having a relative with breast cancer at any age can increase risk.[
Albright et al. addressed how affected third-degree relatives (TDRs) can contribute to an individual's breast cancer risk.[
One of the largest studies of twins ever conducted examined 80,309 monozygotic twins and 123,382 dizygotic twins. This study had a heritability estimate of 31% for breast cancer (95% CI, 11%–51%).[
Benign breast disease, mammographic density, and background parenchymal enhancement
Benign breast disease (BBD)
- BBD is a broad group of conditions characterized by non-cancerous changes in breast tissue. BBD can be divided into three categories: nonproliferative lesions, proliferative lesions without atypia, and atypical hyperplasias. BBD is a consistent risk factor for breast cancer in the general population.[
15 ,16 ] - BBD is also an important risk factor in women who have high breast cancer susceptibility due to family histories of cancer or pathogenic variants in breast cancer risk genes. For example, a study of 17,154 women found that women with a history of BBD have an increased risk of breast cancer that is independent of their underlying familial and genetic risks.[
17 ] However, breast cancer risk associated with personal histories of BBD did not vary between women with BRCA1 pathogenic variants (RR, 1.64; 95% CI, 1.04–2.58), women with BRCA2 pathogenic variants (RR, 1.34; 95% CI, 0.78–2.3), and women who only had family histories of breast cancer (RR, 1.31; 95% CI, 1.13–1.53). In women with high breast cancer susceptibility, BBD can further increase breast cancer risk, because it multiplies their underlying familial and genetic risks.
Mammographic density
- Women with dense breast tissue (assessed by mammogram) also have an increased risk of developing breast cancer.[
15 ,18 ,19 ] Studies have shown that breast density likely has a genetic etiology.[20 ,21 ,22 ] - A systematic review reported that women who had dense breast tissue and an FDR with breast cancer had an increased chance of developing breast cancer.[
23 ] Two retrospective studies also investigated the association between mammographic density and breast cancer risk in BRCA1 and BRCA2 carriers.[24 ,25 ] These retrospective studies had samples of 206 and 691 BRCA pathogenic variant carriers. In these studies, 96 and 248 women developed breast cancer, respectively.[24 ,25 ] The studies found that mammographic density is an independent risk factor for breast cancer in both BRCA1 and BRCA2 pathogenic variant carriers. Associations between breast density and breast cancer risk were similar to those observed in the general population (RR, 2.30 for density ≥50% vs. <50%).
Background parenchymal enhancement (BPE)
- Like breast density (assessed by mammogram), BPE (assessed by breast magnetic resonance imaging [MRI]) may increase breast cancer risk. Data have shown that moderate BPE (odds ratio [OR], 1.6; 95% CI, 1.0–2.6) and mild BPE (OR, 2.1; 95% CI, 1.5–3.0) can increase breast cancer risk in women with high breast cancer susceptibility. However, an association between mild/moderate BPE and breast cancer risk was not found in women with average breast cancer susceptibility.[
26 ]
Parity, age at first birth, and breastfeeding
Parity
- A large prospective study analyzed the relationship between parity and breast cancer risk in female BRCA1 and BRCA2 carriers. Results showed that parity affected breast cancer risk in BRCA1 and BRCA2 carriers differently. Breast cancer risk increased in uniparous BRCA1 carriers and parous BRCA2 carriers.[
27 ] In BRCA1 carriers, there was no overall association between parity and breast cancer risk when compared with nulliparity and breast cancer risk. Uniparous BRCA1 carriers were at an increased risk of breast cancer in the prospective analysis (HRprospective, 1.69; 95% CI, 1.09–2.62) when compared with nulliparous BRCA1 carriers. The results also suggested that uniparous women who breastfed may have decreased breast cancer risk when compared with those who did not breastfeed. In BRCA2 carriers, being parous was associated with a 33% increase in breast cancer risk (HRcombined, 1.33; 95% CI, 1.05–1.69). Multiparity did not decrease breast cancer risk in BRCA2 carriers, unless they had at least four full-term pregnancies (HRcombined, 0.72; 95% CI, 0.54–0.98).
Age at first birth
- In the general population, breast cancer risk increases when women have early menarche and/or late menopause. Breast cancer risk decreases when a woman's first full-term pregnancy occurs at a young age. However, these risk factors can affect women with high breast cancer susceptibility differently than women in the general population. BRCA1 and BRCA2 pathogenic variant carriers who become pregnant prior to age 30 years may have increased breast cancer risk. This effect is even more significant in BRCA1 pathogenic variant carriers.[
28 ,29 ,30 ]BRCA1 and BRCA2 pathogenic variant carriers who developed breast cancer during pregnancy or became pregnant after developing breast cancer did not experience adverse survival outcomes.[31 ]
Breastfeeding
- Breastfeeding can reduce breast cancer risk in BRCA1 (but not BRCA2) pathogenic variant carriers.[
32 ] Breastfeeding for long periods of time was associated with decreased breast cancer risk in BRCA1 carriers (P -trend = .0003).[27 ]
Reproductive history can also affect a woman's risk for ovarian cancer and endometrial cancer. For more information, see the
Contraceptives
Breast cancer risk is one of the factors to consider when prescribing contraceptives, which assist with pregnancy control, abnormal bleeding, and other gynecological symptoms. Oral contraceptives (OCs) may slightly increase breast cancer risk in long-term users, but this appears to be a short-term effect.[
Some studies show that OC use does not further increase breast cancer risk in women with high breast cancer susceptibility. For example, a meta-analysis with data from 54 studies showed that women with family histories of breast cancer did not have increased breast cancer risk from OC use.[
Some studies also suggest that the year an OC was made and a woman's age when beginning OC use may matter. For example, OCs made before 1975 are associated with increased breast cancer risk in BRCA1/2 carriers (summary relative risk [SRR], 1.47; 95% CI, 1.06–2.04).[
Other contraceptive methods have not been studied in women with pathogenic variants in breast cancer risk genes. However, studies have investigated associations between intrauterine devices and breast cancer risk in the general population. A meta-analysis and systematic review of seven studies examined the effect of the levonorgestrel-releasing intrauterine system (LNG-IUS) on breast cancer risk. The meta-analysis included studies that controlled for family history of breast cancer, but associations were not separately evaluated or stratified by family history of breast cancer. In LNG-IUS users, breast cancer risk increased in all women (OR, 1.16; 95% CI, 1.06–1.28), in women younger than 50 years (OR, 1.12; 95% CI, 1.02–1.22), and in women 50 years and older (OR, 1.52; 95% CI, 1.34–1.72).[
Hormone replacement therapy
Both observational studies and randomized clinical trials have examined the association between postmenopausal HRT and breast cancer. Short-term use of HRT for treatment of postmenopausal symptoms appears to confer little or no breast cancer risk.[
Among women with family histories of breast cancer, the associations between HRT and breast cancer risk have not been consistent. Some studies suggested risk was particularly elevated among women with family histories of breast cancer, while others did not report an interaction between these factors.[
The effect of HRT on breast cancer risk among carriers of BRCA1 and BRCA2 pathogenic variants has been studied in the context of bilateral risk-reducing oophorectomy. Short-term HRT use does not seem to alter an oophorectomy's protective effect on breast cancer risk.[
HRT use may also increase a woman's chance of developing endometrial cancer. For more information, see the
Radiation exposure
Radiation exposure can increase an individual's breast cancer risk. This is demonstrated by the survivors of the atomic bombings in Hiroshima and Nagasaki and by women who have received therapeutic radiation treatments to the chest and upper body. However, it is unclear how much radiation exposure affects breast cancer risk in women with high breast cancer susceptibility.
Early data suggested that carriers of BRCA1 and BRCA2 pathogenic variants may have increased sensitivity to radiation, which may contribute to cancer susceptibility.[
It is possible that radiation exposure from diagnostic procedures, including mammography, poses a greater risk to women with high breast cancer susceptibility than to women who are at average risk of developing breast cancer. Therapeutic radiation could also increase cancer risk in women with high breast cancer susceptibility. However, a cohort study of BRCA1 and BRCA2 pathogenic variant carriers treated with breast-conserving therapy did not show evidence of increased radiation sensitivity in participants. Sequelae were not observed in the breasts, lungs, or bone marrow of BRCA carriers.[
Conversely, tumors in women with pathogenic variants in breast cancer risk genes may be more responsive to radiation treatment than tumors in women at average breast cancer risk. Studies examining the impact of radiation exposure in carriers of BRCA1 and BRCA2 pathogenic variants have had conflicting results.[
A retrospective cohort study estimated the effect of adjuvant radiation therapy (for primary breast cancer) on CBC risk in BRCA1 and BRCA2 carriers (N, 691; median follow-up period, 8.6 y).[
Alcohol and smoking
The risk of breast cancer increases by approximately 10% for each 10 g of daily alcohol intake (approximately one drink or less) in the general population.[
Recent studies have evaluated the association between alcohol consumption, tobacco smoking, and breast cancer risk in individuals with BRCA1/2 pathogenic variants or family histories of breast cancer. One study evaluated if tobacco smoking and alcohol consumption are associated with increased breast cancer risk in BRCA1 and BRCA2 carriers using pooled data from an international cohort.[
Physical activity
Increased physical activity has been associated with reduced breast cancer risk in most epidemiological studies. This risk reduction has also been seen in studies of female BRCA1 or BRCA2 pathogenic variant carriers. For example, one study reported a 38% reduction in premenopausal breast cancer risk from moderate physical activity (OR for the top quartile of physical activity compared with the lowest level, 0.62; 95% CI, 0.40–0.96).[
Risk Factors for Ovarian Cancer
Refer to the PDQ summary on
Age
Ovarian cancer incidence rises in a linear fashion from age 30 years to age 50 years and continues to increase, though at a slower rate, thereafter. Before age 30 years, the risk of developing epithelial ovarian cancer is remote, even in hereditary cancer families.[
Family history including inherited cancer genes
Although reproductive, demographic, and lifestyle factors affect risk of ovarian cancer, the single greatest ovarian cancer risk factor is a family history of the disease. A large meta-analysis of 15 published studies estimated an OR of 3.1 for the risk of ovarian cancer associated with at least one FDR with ovarian cancer.[
Reproductive history
Nulliparity is consistently associated with an increased risk of ovarian cancer, including among carriers of BRCA/BRCA2 pathogenic variants, yet a meta-analysis identified a risk reduction only in women with four or more live births.[
Surgical history
Bilateral tubal ligation and hysterectomy are associated with reduced ovarian cancer risk,[
Oral contraceptives (OCs)
Use of OCs for 4 or more years is associated with an approximately 50% reduction in ovarian cancer risk in the general population.[
Risk Factors for Endometrial Cancer
Refer to the PDQ summary on
Age
Age is an important risk factor for endometrial cancer. Most women with endometrial cancer are diagnosed after menopause. Only 15% of women are diagnosed with endometrial cancer before age 50 years, and fewer than 5% are diagnosed before age 40 years.[
Family history including inherited cancer genes
Although the hyperestrogenic state is the most common predisposing factor for endometrial cancer, family history also plays a significant role in a woman's risk for disease. Approximately 3% to 5% of uterine cancer cases are attributable to a hereditary cause,[
Non-Lynch syndrome genes may also contribute to endometrial cancer risk. In an unselected endometrial cancer cohort undergoing multigene panel testing, approximately 3% of patients tested positive for a germline pathogenic variant in non-Lynch syndrome genes, including CHEK2, APC, ATM, BARD1, BRCA1, BRCA2, BRIP1, NBN, PTEN, and RAD51C.[
Reproductive history
Reproductive factors such as multiparity, late menarche, and early menopause decrease the risk of endometrial cancer because of the lower cumulative exposure to estrogen and the higher relative exposure to progesterone.[
Hormones
Hormonal factors that increase the risk of type I endometrial cancer are better understood. All endometrial cancers share a predominance of estrogen relative to progesterone. Prolonged exposure to estrogen or unopposed estrogen increases the risk of endometrial cancer. Endogenous exposure to estrogen can result from obesity, polycystic ovary syndrome, and nulliparity, while exogenous estrogen can result from taking unopposed estrogen or tamoxifen. Unopposed estrogen increases the risk of developing endometrial cancer by twofold to twentyfold, proportional to the duration of use.[
Autosomal Dominant Inheritance of Breast and Gynecologic Cancer Predisposition
Autosomal dominant inheritance of breast and gynecologic cancers is characterized by transmission of cancer predisposition from generation to generation, through either the mother's or the father's side of the family, with the following characteristics:
- Inheritance risk of 50%. When a parent carries an autosomal dominant genetic predisposition, each child has a 50:50 chance of inheriting the predisposition. Although the risk of inheriting the predisposition is 50%, not everyone with the predisposition will develop cancer because of incomplete penetrance and/or gender-restricted or gender-related expression.
- Both males and females can inherit and transmit an autosomal dominant cancer predisposition. A male who inherits a cancer predisposition can still pass the altered gene on to his sons and daughters.
Breast and ovarian cancer are components of several autosomal dominant cancer syndromes. The syndromes most strongly associated with both cancers are the syndromes associated with BRCA1 or BRCA2 pathogenic variants. Breast cancer is also a common feature of
Germline pathogenic variants in the genes responsible for these autosomal dominant cancer syndromes produce different clinical phenotypes of characteristic malignancies and, in some instances, associated nonmalignant abnormalities.
The family characteristics that suggest hereditary cancer predisposition include the following:
- Multiple cancers within a family.
- Cancers typically occur at an earlier age than in sporadic cases (defined as cases not associated with genetic risk).
- Two or more primary cancers in a single individual. These could be multiple primary cancers of the same type (e.g., bilateral breast cancer) or primary cancer of different types (e.g., breast cancer and ovarian cancer in the same individual or endometrial and colon cancer in the same individual).
- Cases of male breast cancer. The inheritance risk for autosomal dominant genetic conditions is 50% for both males and females, but the differing penetrance of the genes may result in some unaffected individuals in the family.
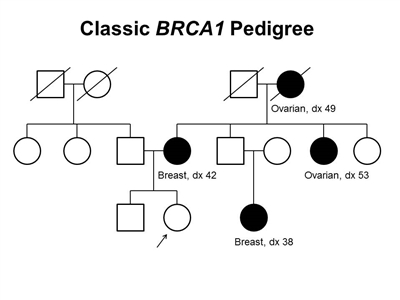
Figure 1. BRCA1 pedigree. This pedigree shows some of the classic features of a family with a BRCA1 pathogenic variant across three generations, including affected family members with breast cancer or ovarian cancer and a young age at onset. BRCA1 families may exhibit some or all of these features. As an autosomal dominant syndrome, a BRCA1 pathogenic variant can be transmitted through maternal or paternal lineages, as depicted in the figure.
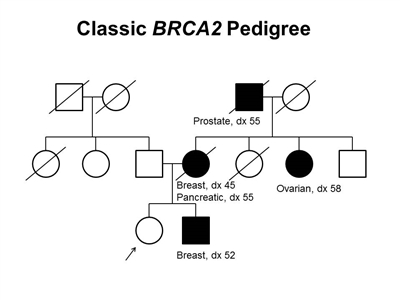
Figure 2. BRCA2 pedigree. This pedigree shows some of the classic features of a family with a BRCA2 pathogenic variant across three generations, including affected family members with breast (including male breast cancer), ovarian, pancreatic, or prostate cancers and a relatively young age at onset. BRCA2 families may exhibit some or all of these features. As an autosomal dominant syndrome, a BRCA2 pathogenic variant can be transmitted through maternal or paternal lineages, as depicted in the figure.
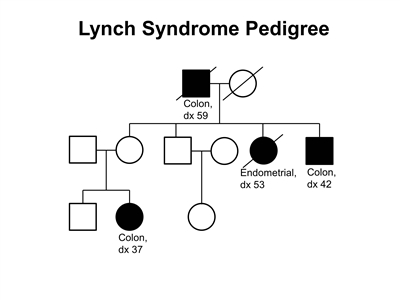
Figure 3. Lynch syndrome pedigree. This pedigree shows some of the classic features of a family with Lynch syndrome, including affected family members with colon cancer or endometrial cancer, a young age at onset in some individuals, and incomplete penetrance. Lynch syndrome families may exhibit some or all of these features. Lynch syndrome families may also include individuals with other gastrointestinal, gynecologic, and genitourinary cancers, or other extracolonic cancers. As an autosomal dominant syndrome, Lynch syndrome can be transmitted through maternal or paternal lineages, as depicted in the figure. Because the cancer risk is not 100%, individuals who have Lynch syndrome may not develop cancer, such as the mother of the female with colon cancer diagnosed at age 37 years in this pedigree (called incomplete penetrance).
There are no pathognomonic features distinguishing breast and ovarian cancers occurring in carriers of BRCA1 or BRCA2 pathogenic variants from those occurring in noncarriers. Breast cancers occurring in carriers of BRCA1 pathogenic variants are more likely to be ER-negative, progesterone receptor (PR)–negative, human epidermal growth factor receptor two (HER2/neu)–negative (i.e., triple-negative breast cancers [TNBC]), and have a basal phenotype. BRCA1-associated ovarian cancers are more likely to be high-grade and of serous histopathology. (Refer to the
Some pathologic features distinguish carriers of Lynch syndrome–associated pathogenic variants from noncarriers. The hallmark feature of endometrial cancers occurring in Lynch syndrome is mismatch repair (MMR) deficiencies, including the presence of microsatellite instability (MSI), and the absence of specific MMR proteins. In addition to these molecular changes, there are also histologic changes including tumor-infiltrating lymphocytes, peritumoral lymphocytes, undifferentiated tumor histology, lower uterine segment origin, and synchronous tumors.
Considerations in Risk Assessment and in Identifying a Family History of Breast and Ovarian Cancer Risk
The accuracy and completeness of family histories must be considered when they are used to assess risk. A reported family history may be erroneous, or a person may be unaware of relatives affected with cancer. In addition, small family sizes and premature deaths may limit the information obtained from a family history. Breast or ovarian cancer on the paternal side of the family usually involves more distant relatives than does breast or ovarian cancer on the maternal side, so information may be more difficult to obtain. When self-reported information is compared with independently verified cases, the sensitivity of a history of breast cancer is relatively high, at 83% to 97%, but lower for ovarian cancer, at 60%.[
Models for Prediction of Breast and Gynecologic Cancer Risk
Models to predict an individual's risk of developing breast and/or gynecologic cancer are available.[
- Calibration: How well the model predicts what will happen. When calibration statistics are close to 1, this means that the predicted value is similar to the actual value.
- Discrimination: How well the model can differentiate between those with and without the outcome. When only case-control data are available, the discrimination of the model (which is often assessed by measuring the area under the receiver operator curve, AUROC or AUC for short) can be assessed but the calibration cannot. An AUC of 1.0 means that the model has perfect discriminatory accuracy. AUCs closer to 0.50 show that the model is poor at discrimination. Generally, an AUC of 0.80 or higher is good to excellent, while AUCs between 0.70 and 0.80 are poor.
There are several items to consider when using models, including (1) time horizon for the prediction, (2) variables included in the model, and (3) whether models can also predict the probability of carrying a pathogenic variant in breast cancer susceptibility genes like BRCA1 and BRCA2.
- Time horizon of models: Most models can predict an individual's lifetime risk of developing a specific cancer over a short time horizon (e.g., 1 year, 5 years, and 10 years). Although some clinical guidelines refer to lifetime risk cutoffs when assessing higher versus lower cancer risks, no model has been validated to predict full lifetime risk, since that would require following cohorts for a lifetime.[
134 ] Using a shorter time horizon improved model performance, particularly for women under age 50 years, since many factors for risk models change over time.[135 ] For example, data from a large family-based cohort (n = 14,657 women; median follow-up of 10 years), showed that the 5-year incidence for breast cancer almost always had a higher specificity (i.e., fewer false positives) than that of lifetime risk from birth. For women aged 20 to 39 years, 5-year risk performed better than lifetime risk from birth. For women aged 40 years or older, receiver-operating characteristic curves were similar or superior for 5-year risk than for lifetime risk in multiple breast cancer models. Classifications based on remaining lifetime risk were inferior to 5-year risk estimates.
- Variables included in models: In addition to a lack of validation for lifetime risk, cancer risk models are limited by the factors added to the models to help predict risk. Unlike risk models for diseases with shorter induction times (e.g., cardiovascular disease), cancer's longer induction times can make updating models (based on known risk factors) lengthy, since prospective validation is needed to calibrate the models. Most breast cancer risk models include established reproductive risk factors for breast cancer (e.g., age at menarche, parity, etc.). Many risk models also include established risk factors like alcohol consumption and body size. Few risk models assess whether cessation or change in risk factors over time lead to a change in cancer risk.
- Prediction of cancer susceptibility genes: In addition, models can predict an individual's likelihood of having a pathogenic variant in BRCA1, BRCA2, or one of the MMR genes associated with Lynch syndrome. Not all models can be applied to all patients. Each model is appropriate only when the patient's characteristics and family history are similar to those from the study population the model was based on. Different models may provide widely varying risk estimates for the same clinical scenario, and validation of these estimates has not been performed for many models.[
131 ,136 ,137 ] For more information, see theModels for prediction of the likelihood of a BRCA1 or BRCA2 pathogenic variant section.
Limitations of risk models: Risk models only use a subset of risk factors for breast, ovarian, and endometrial cancer risk. Additionally, risk models are limited by moderate discrimination for these cancer types. Moderate discrimination means that when clinical cutoffs are used to define high- and low-risk individuals (e.g., individuals with >20% lifetime risk are defined as high-risk), people will be misclassified. This means that there will be both false positives (people at lower risk who follow high-risk protocols) and false negatives (people at higher risk who follow low-risk protocols).
Breast cancer risk assessment models
In general, breast cancer risk assessment models are designed for two types of populations: (1) women without pathogenic variants in breast cancer susceptibility genes or strong family histories of breast/ovarian cancer, and (2) women at higher risk because of personal or family histories of breast/ovarian cancer.[
Models of the first type designed for women (e.g., the Gail model, which is the basis for the Breast Cancer Risk Assessment Tool [BCRAT] [
Models designed for women at higher risk require more detailed information about personal and family cancer histories of breast and ovarian cancers, including ages at onset of cancer and/or carrier status of specific breast cancer-susceptibility alleles. The genetic factors used by the latter models differ, with some assuming one risk locus (e.g., the Claus model [
The models also differ in whether they include information about nongenetic risk factors. Three models (Gail/BCRAT, Pfeiffer,[
Breast cancer risk models have limited the ability to discriminate between individuals who are affected or unaffected with cancer. A model with high discrimination would be close to 1, and a model with little discrimination would be close to 0.5. Model discrimination is rarely above an AUC of 0.70.[
In the United States, the BRCAPRO, Claus,[
In addition to statistical and regression-based models, risk-assessment models are being developed based on artificial intelligence (AI), using imaging (primarily from mammography) and other clinical data from the electronic health record. Risk-assessment based on machine learning and AI algorithms (when applied to mammographic images) have produced AUCs in a similar or even higher range than some of the pedigree and regression-based risk models.[
Additional considerations for clinical use of breast cancer risk assessment models
The Gail model is the basis for the
- Women who do not adhere to mammography screening recommendations.[
155 ,156 ] - Women in the highest-risk strata (e.g., those with breast cancer family histories, particularly if FDRs are older when diagnosed with breast cancer).[
158 ]
The Gail/BCRAT model is valid for women aged 35 years and older. The model was primarily developed for White women.[
Generally, the Gail/BCRAT model should not be the sole model used for families with one or more of the following characteristics:
- Multiple affected individuals with breast cancer or ovarian cancer (especially when one or more breast cancers are diagnosed before age 50 y).
- A woman with both breast and ovarian cancer.
- Ashkenazi Jewish ancestry with at least one case of breast or ovarian cancer (as these families are more likely to have a hereditary cancer susceptibility syndrome).
Commonly used models that incorporate family history include the IBIS, BOADICEA/CanRisk, and BRCAPRO models. The IBIS/Tyrer-Cuzick model incorporates both genetic and nongenetic factors.[
In addition, readily available models that provide information about an individual woman's risk in relation to the population-level risk depending on her risk factors may be useful in a clinical setting (e.g.,
Although most breast cancer risk models have been shown to be well calibrated overall, model performance can be different for subgroups of women. In particular, independent, prospective validation of risk models for women who tested negative for BRCA1 or BRCA2 pathogenic variants supported that the most commonly used clinical risk models underpredicted risk for this group of women.[
Risk models in older individuals: As individuals age, the chance to have competing risks from other outcomes increases (e.g., cardiovascular disease). Some risk models incorporate the concept of competing risk into their calculations (e.g., BCRAT), while others do not (e.g., BOADICEA/CanRisk). Differences that occur due to competing risk are particularly important to consider, especially in older women with other comorbidities.
Ovarian cancer risk assessment models
Model development for prediction of ovarian cancer risk has been similar to that of breast cancer risk models with pedigree-based models and nonpedigree-based models.
Endometrial cancer risk assessment models
Endometrial cancer risk models also can be divided into regression-based models, pedigree-based models, and AI-based models. The Pfeiffer model has been used to predict endometrial cancer risk in the general population.[
Regression-based models differ from pedigree-based models, which require detailed information on the number of relatives with cancer, types of cancer, and ages of cancer diagnoses in family members. MMRpredict, PREMM5 (PREdiction Model for gene Mutations), and MMRpro are three quantitative predictive models used to identify individuals who may potentially have Lynch syndrome.[
AI-based models have been used for risk-stratification and prognosis in endometrial cancer cases, but they have not been used to predict risk of endometrial cancer onset.[
Models for Predicting the Likelihood of aBRCA1/BRCA2Pathogenic Variant
Many models have been developed to predict the probability of identifying germline BRCA1/BRCA2 pathogenic variants in individuals or families. These models include those using logistic regression,[
In addition to BOADICEA, BRCAPRO is commonly used for genetic counseling in the clinical setting. BRCAPRO and BOADICEA predict the probability of being a carrier and produce estimates of breast cancer risk (refer to
BOADICEA is a polygenetic model that uses complex segregation analysis to examine both breast cancer risk and the probability of having a BRCA1 or BRCA2 pathogenic variant.[
The performance of the models can vary in specific ethnic groups. The BRCAPRO model appeared to best fit a series of French Canadian families.[
The power of several of the models has been compared in different studies.[
| | Myriad Prevalence Tables[ |
BRCAPRO[ |
BOADICEA (now CanRisk)[ |
Tyrer-Cuzick[ |
|---|---|---|---|---|
| AJ = Ashkenazi Jewish; BOADICEA = Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm; FDR = first-degree relatives; SDR = second-degree relatives. | ||||
| Method | Empiric data from Myriad Genetics based on personal and family history reported on requisition forms | Statistical model, assumes autosomal dominant inheritance | Statistical model, assumes polygenic risk | Statistical model, assumes autosomal dominant inheritance |
| Features of the model | Probandmay or may not have breast or ovarian cancer | Proband may or may not have breast or ovarian cancer | Proband may or may not have breast or ovarian cancer | Proband must beunaffected |
| Considers age of breast cancer diagnosis as <50 y, >50 y | Considers exact age at breast and ovarian cancer diagnosis | Considers exact age at breast and ovarian cancer diagnosis | Also includes reproductive factors and body mass index to estimate breast cancer risk | |
| Considers breast cancer in ≥1 affected relative only if diagnosed <50 y | Considers prior genetic testing in family (i.e.,BRCA1/BRCA2 pathogenic variant–negative relatives) | Includes allFDRandSDRwith and without cancer | ||
| Considers ovarian cancer in ≥1 relative at any age | Considers oophorectomy status | Includes AJ ancestry | ||
| Includes AJ ancestry | Includes all FDR and SDR with and without cancer | |||
| Very easy to use | Includes AJ ancestry | |||
| Limitations | Simplified/limited consideration of family structure | Requires computer software and time-consuming data entry | Requires computer software and time-consuming data entry | Designed for individuals unaffected with breast cancer |
| Incorporates only FDR and SDR; may need to change proband to best capture risk and to account for disease in the paternal lineage | ||||
| May overestimate risk in bilateral breast cancer[ |
||||
| Early age of breast cancer onset | May perform better in White populations than in racial and ethnic minority populations[ |
Incorporates only FDR and SDR; may need to change proband to best capture risk | ||
| May underestimate risk ofBRCApathogenic variant in high-grade serous ovarian cancers but overestimate the risk for other histologies[ |
||||
Genetic testing for BRCA1 and BRCA2 pathogenic variants has been available to the public since 1996. As more individuals have undergone testing, risk assessment models have improved. This, in turn, gives providers better data to estimate an individual patient's risk of carrying a pathogenic variant, but risk assessment continues to be an art. There are factors that might limit the ability to provide an accurate risk assessment (i.e., small family size, paucity of women, or ethnicity) including the specific circumstances of the individual patient (such as history of disease or risk-reducing surgeries).
Considerations When Conducting Genetic Testing
Indications for hereditary breast and gynecologic cancers genetic testing
Several professional organizations and expert panels—including the American Society of Clinical Oncology,[
In 2019, the American Society of Breast Surgeons published a recommendation to make genetic testing for "BRCA1/BRCA2, and PALB2, with other genes as appropriate for the clinical scenario and family history" available to all breast cancer patients.[
Other studies have also found that the NCCN criteria have good sensitivity when predicting BRCA1/BRCA2 variants; however, less is known about many other genes. For example, one study showed that the NCCN criteria were able to detect 88.9% of the BRCA1/BRCA2 pathogenic variant carriers [
As the cost of genetic testing continues to decrease, there is a need for unbiased evidence to guide indications for testing, including the cost-benefit impact on screening, prevention, and treatment. Efforts to generate less biased evidence include a single institution study of 3,907 unselected women with breast cancer tested for nine breast cancer genes, including BRCA1/BRCA2, ATM, CDH1, CHEK2, NF1, PALB2, PTEN, and TP53.[
Another study to assess frequency of pathogenic or likely pathogenic variants among breast cancer patients included a nested case-control study conducted through the WHI cohort among women with (cases) and without (controls) invasive breast cancer. Participants were tested for pathogenic or likely pathogenic variants in ten breast cancer–associated genes, including BRCA1/BRCA2.[
Benefits of offering genetic testing at the time of cancer diagnosis
At the time of a new cancer diagnosis, genetic testing for inherited cancer predisposition may guide patient care including decisions about surgery, chemotherapy and other biologics, and radiation treatment.[
Breast cancer diagnosis
Benefits of offering genetic testing at the time of breast cancer diagnosis include, but are not limited to, the following:
- Surgery: The identification of inherited susceptibility to breast cancer may influence surgical treatment decisions. As an example, the high risk of a second primary breast cancer among BRCA pathogenic variant carriers, particularly those diagnosed at an early age, may influence their decision to choose a bilateral mastectomy (versus a lumpectomy or unilateral/subtotal mastectomy) for surgical treatment of their breast cancer.[
223 ] Discussion of RRSO is indicated,[224 ] and referral to a gynecologic provider may be considered. - Chemotherapy and other biologics: Medical treatments may be guided by the identification of a pathogenic variant in an inherited cancer predisposing gene. As an example, among BRCA pathogenic variant carriers, breast cancer treatment may include the use of platinum-based agents.[
225 ] Furthermore, novel agents such as PARP inhibitors may be used in the treatment of metastatic breast cancer.[226 ] - Radiation therapy: Decisions about the use of radiation treatment may be guided by the presence of a pathogenic variant in an inherited breast cancer susceptibility gene. In particular, the poorer wound healing in irradiated breasts is an important consideration for those who may consider risk-reducing mastectomy with reconstruction. As an example, individuals with a pathogenic variant in TP53 may experience higher risks from radiation, including increased risks for subsequent new cancers.[
227 ,228 ] Thus, identification of TP53 carriers in the context of an active breast cancer diagnosis may influence radiation treatment decisions and reconstruction options.
Ovarian cancer diagnosis
Benefits of offering genetic testing at the time of ovarian cancer diagnosis include, but are not limited to, the following:
- Surgery: In most cases, the decision for ovarian cancer surgery is made on the basis of an adnexal mass or abdominal symptoms. When possible, considering the likelihood of a heritable genetic variant at the time of diagnosis may add value to surgical decision-making. The identification of inherited susceptibility to ovarian/fallopian tube cancer may influence surgical treatment decisions. For a questionable adnexal mass in a younger woman who is at risk of carrying a pathogenic variant of a highly penetrant ovarian cancer gene, knowledge of this information may help guide a decision for risk-reducing or therapeutic surgery.[
229 ,230 ] For women who may be considering fertility preservation surgery, genetic knowledge may motivate consideration of bilateral salpingo-oophorectomy, and in the case of carriers of BRCA1 pathogenic variants, a more detailed discussion regarding aggressive uterine cancer risk. - Chemotherapy and other biologics: First-line chemotherapy for ovarian cancer still relies on a backbone of platinum and taxane chemotherapy. Current treatment options for optimally resected stage III ovarian carcinoma include intravenous (IV) chemotherapy, dose-dense IV chemotherapy, and a combination of IV paclitaxel plus intraperitoneal (IP) cisplatin, followed by IP paclitaxel 1 week later. Carriers of BRCA1 and BRCA2 pathogenic variants are considered more platinum sensitive, with longer progression-free survival times compared with BRCA1 and BRCA2 wild-type patients,[
231 ,232 ] so it is unclear whether a particular treatment strategy is driven more by antiangiogenesis effects, peritoneal dose intensity, or platinum dose intensity. The advent of PARP as a biologic target (in combination with chemotherapy or as maintenance) may also increase the armory of first-line treatment of ovarian cancer.[233 ] (Refer to theOvarian Cancer Treatment Strategies section in BRCA1 and BRCA2: Cancer Risks and Management for more information about PARP inhibitors in ovarian cancer treatment.)
Endometrial cancer diagnosis
Benefits of offering genetic testing at the time of endometrial cancer diagnosis include, but are not limited to, the following:
- Surgery: The most common treatment for a newly diagnosed endometrial cancer includes hysterectomy with removal of the ovaries and fallopian tubes, as well as assessment of lymph nodes.[
234 ] An exception to this practice might apply to a younger woman who wishes to retain fertility or retain her adnexa. IHC of endometrial sampling may allow for an assessment of the likelihood of a heritable genetic variant at the time of diagnosis, which may add value to the surgical decision-making process. For a young woman who is found to have Lynch syndrome, knowledge of this information may help guide a decision for hormonal management of endometrial cancer to allow future childbearing, or RRSO if her risk of ovarian cancer is deemed high enough on the basis of a specific genetic variant. For a young woman who is found to carry a pathogenic variant in BRCA1/BRCA2, or one of the other homologous recombination deficiencies increasing ovarian cancer risk, she may wish to decide between salpingo-oophorectomy or, at least, salpingectomy. - Chemotherapy and other biologics: Immune checkpoint inhibitors are now approved for use in endometrial cancers that have MSI or MMR deficiency.[
235 ] While MSI and MMR status can be assessed at either the time of diagnosis or recurrent disease, it may be beneficial to perform tumor testing at diagnosis with the primary pathology processing, usually at the time of hysterectomy.
Multigene (panel) testing
Since the availability of next-generation sequencing and the Supreme Court of the United States ruling that human genes cannot be patented, several clinical laboratories now offer genetic testing through multigene panels at a cost comparable to that of single-gene testing. Even testing for BRCA1 and BRCA2 is a limited panel test of two genes. Approximately 25% of all ovarian/fallopian tube/peritoneal cancers are caused by a heritable genetic condition. Of these, about one-quarter (6% of all ovarian/fallopian tube/peritoneal cancers) are caused by genes other than BRCA1 and BRCA2, including many genes associated with the Fanconi anemia pathway or otherwise involved with homologous recombination.[
In general, multigene panel testing increases the yield of non-BRCA pathogenic variants across a variety of populations.[
Multi-gene panel testing was conducted as part of two large efforts led by the worldwide Breast Cancer Association Consortium (BCAC) [
NCCN recommends that women diagnosed with TNBC undergo BRCA1/BRCA2, CDH1, PALB2, PTEN, STK11, and TP53 testing to guide treatment decisions at any age.[
Multi-gene panel testing studies were conducted in women from the United States who had African ancestry, and results showed that certain genes were associated with increased breast cancer risk in this population. These genes were similar to the breast cancer risk genes found in individuals from the United States with European ancestry. A case-control study of 10,047 women with African ancestry found a pathogenic variant frequency of 10.3% in those with ER-negative breast cancer, 5.2% in those with ER-positive breast cancer, and 2.3% in those without breast cancer. BRCA1 (OR, 47), BRCA2 (OR, 7.25) and PALB2 (OR, 8.54) were associated with the highest breast cancer risks.[
There are caveats of multigene testing. Genes identified as part of multigene panel testing can be associated with varied breast cancer risk or confer no known risk.[
(Refer to the
References:
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025.
Available online . Last accessed January 16, 2025. - Ravdin PM, Cronin KA, Howlader N, et al.: The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 356 (16): 1670-4, 2007.
- Quante AS, Herz J, Whittemore AS, et al.: Assessing absolute changes in breast cancer risk due to modifiable risk factors. Breast Cancer Res Treat 152 (1): 193-7, 2015.
- Feuer EJ, Wun LM, Boring CC, et al.: The lifetime risk of developing breast cancer. J Natl Cancer Inst 85 (11): 892-7, 1993.
- Yang Q, Khoury MJ, Rodriguez C, et al.: Family history score as a predictor of breast cancer mortality: prospective data from the Cancer Prevention Study II, United States, 1982-1991. Am J Epidemiol 147 (7): 652-9, 1998.
- Colditz GA, Willett WC, Hunter DJ, et al.: Family history, age, and risk of breast cancer. Prospective data from the Nurses' Health Study. JAMA 270 (3): 338-43, 1993.
- Slattery ML, Kerber RA: A comprehensive evaluation of family history and breast cancer risk. The Utah Population Database. JAMA 270 (13): 1563-8, 1993.
- Johnson N, Lancaster T, Fuller A, et al.: The prevalence of a family history of cancer in general practice. Fam Pract 12 (3): 287-9, 1995.
- Pharoah PD, Day NE, Duffy S, et al.: Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer 71 (5): 800-9, 1997.
- Bevier M, Sundquist K, Hemminki K: Risk of breast cancer in families of multiple affected women and men. Breast Cancer Res Treat 132 (2): 723-8, 2012.
- Kharazmi E, Chen T, Narod S, et al.: Effect of multiplicity, laterality, and age at onset of breast cancer on familial risk of breast cancer: a nationwide prospective cohort study. Breast Cancer Res Treat 144 (1): 185-92, 2014.
- Reiner AS, Sisti J, John EM, et al.: Breast Cancer Family History and Contralateral Breast Cancer Risk in Young Women: An Update From the Women's Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol 36 (15): 1513-1520, 2018.
- Albright FS, Kohlmann W, Neumayer L, et al.: Population-based relative risks for specific family history constellations of breast cancer. Cancer Causes Control 30 (6): 581-590, 2019.
- Mucci LA, Hjelmborg JB, Harris JR, et al.: Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 315 (1): 68-76, 2016.
- Chen J, Pee D, Ayyagari R, et al.: Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst 98 (17): 1215-26, 2006.
- Dupont WD, Page DL, Parl FF, et al.: Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med 331 (1): 10-5, 1994.
- Zeinomar N, Phillips KA, Daly MB, et al.: Benign breast disease increases breast cancer risk independent of underlying familial risk profile: Findings from a Prospective Family Study Cohort. Int J Cancer 145 (2): 370-379, 2019.
- Boyd NF, Byng JW, Jong RA, et al.: Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst 87 (9): 670-5, 1995.
- Byrne C, Schairer C, Wolfe J, et al.: Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst 87 (21): 1622-9, 1995.
- Pankow JS, Vachon CM, Kuni CC, et al.: Genetic analysis of mammographic breast density in adult women: evidence of a gene effect. J Natl Cancer Inst 89 (8): 549-56, 1997.
- Boyd NF, Lockwood GA, Martin LJ, et al.: Mammographic densities and risk of breast cancer among subjects with a family history of this disease. J Natl Cancer Inst 91 (16): 1404-8, 1999.
- Vachon CM, King RA, Atwood LD, et al.: Preliminary sibpair linkage analysis of percent mammographic density. J Natl Cancer Inst 91 (20): 1778-9, 1999.
- Vachon CM, van Gils CH, Sellers TA, et al.: Mammographic density, breast cancer risk and risk prediction. Breast Cancer Res 9 (6): 217, 2007.
- Mitchell G, Antoniou AC, Warren R, et al.: Mammographic density and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Res 66 (3): 1866-72, 2006.
- Ramón Y Cajal T, Chirivella I, Miranda J, et al.: Mammographic density and breast cancer in women from high risk families. Breast Cancer Res 17: 93, 2015.
- Thompson CM, Mallawaarachchi I, Dwivedi DK, et al.: The Association of Background Parenchymal Enhancement at Breast MRI with Breast Cancer: A Systematic Review and Meta-Analysis. Radiology 292 (3): 552-561, 2019.
- Terry MB, Liao Y, Kast K, et al.: The Influence of Number and Timing of Pregnancies on Breast Cancer Risk for Women With BRCA1 or BRCA2 Mutations. JNCI Cancer Spectr 2 (4): pky078, 2018.
- Johannsson O, Loman N, Borg A, et al.: Pregnancy-associated breast cancer in BRCA1 and BRCA2 germline mutation carriers. Lancet 352 (9137): 1359-60, 1998.
- Jernström H, Lerman C, Ghadirian P, et al.: Pregnancy and risk of early breast cancer in carriers of BRCA1 and BRCA2. Lancet 354 (9193): 1846-50, 1999.
- Friebel TM, Domchek SM, Rebbeck TR: Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst 106 (6): dju091, 2014.
- Valentini A, Lubinski J, Byrski T, et al.: The impact of pregnancy on breast cancer survival in women who carry a BRCA1 or BRCA2 mutation. Breast Cancer Res Treat 142 (1): 177-85, 2013.
- Jernström H, Lubinski J, Lynch HT, et al.: Breast-feeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 96 (14): 1094-8, 2004.
- Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 347 (9017): 1713-27, 1996.
- Iodice S, Barile M, Rotmensz N, et al.: Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer 46 (12): 2275-84, 2010.
- Schrijver LH, Olsson H, Phillips KA, et al.: Oral Contraceptive Use and Breast Cancer Risk: Retrospective and Prospective Analyses From a BRCA1 and BRCA2 Mutation Carrier Cohort Study. JNCI Cancer Spectr 2 (2): pky023, 2018.
- Huber D, Seitz S, Kast K, et al.: Use of oral contraceptives in BRCA mutation carriers and risk for ovarian and breast cancer: a systematic review. Arch Gynecol Obstet 301 (4): 875-884, 2020.
- Kotsopoulos J, Lubinski J, Moller P, et al.: Timing of oral contraceptive use and the risk of breast cancer in BRCA1 mutation carriers. Breast Cancer Res Treat 143 (3): 579-86, 2014.
- Conz L, Mota BS, Bahamondes L, et al.: Levonorgestrel-releasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet Gynecol Scand 99 (8): 970-982, 2020.
- Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 350 (9084): 1047-59, 1997.
- Gorsky RD, Koplan JP, Peterson HB, et al.: Relative risks and benefits of long-term estrogen replacement therapy: a decision analysis. Obstet Gynecol 83 (2): 161-6, 1994.
- Writing Group for the Women's Health Initiative Investigators: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288 (3): 321-33, 2002.
- Chlebowski RT, Hendrix SL, Langer RD, et al.: Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA 289 (24): 3243-53, 2003.
- Beral V; Million Women Study Collaborators: Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362 (9382): 419-27, 2003.
- Anderson GL, Limacher M, Assaf AR, et al.: Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291 (14): 1701-12, 2004.
- Schuurman AG, van den Brandt PA, Goldbohm RA: Exogenous hormone use and the risk of postmenopausal breast cancer: results from The Netherlands Cohort Study. Cancer Causes Control 6 (5): 416-24, 1995.
- Steinberg KK, Thacker SB, Smith SJ, et al.: A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA 265 (15): 1985-90, 1991.
- Sellers TA, Mink PJ, Cerhan JR, et al.: The role of hormone replacement therapy in the risk for breast cancer and total mortality in women with a family history of breast cancer. Ann Intern Med 127 (11): 973-80, 1997.
- Stanford JL, Weiss NS, Voigt LF, et al.: Combined estrogen and progestin hormone replacement therapy in relation to risk of breast cancer in middle-aged women. JAMA 274 (2): 137-42, 1995.
- Colditz GA, Egan KM, Stampfer MJ: Hormone replacement therapy and risk of breast cancer: results from epidemiologic studies. Am J Obstet Gynecol 168 (5): 1473-80, 1993.
- Rebbeck TR, Friebel T, Wagner T, et al.: Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 23 (31): 7804-10, 2005.
- Kotsopoulos J, Gronwald J, Karlan BY, et al.: Hormone Replacement Therapy After Oophorectomy and Breast Cancer Risk Among BRCA1 Mutation Carriers. JAMA Oncol 4 (8): 1059-1065, 2018.
- Gordhandas S, Norquist BM, Pennington KP, et al.: Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol 153 (1): 192-200, 2019.
- Helzlsouer KJ, Harris EL, Parshad R, et al.: Familial clustering of breast cancer: possible interaction between DNA repair proficiency and radiation exposure in the development of breast cancer. Int J Cancer 64 (1): 14-7, 1995.
- Helzlsouer KJ, Harris EL, Parshad R, et al.: DNA repair proficiency: potential susceptibility factor for breast cancer. J Natl Cancer Inst 88 (11): 754-5, 1996.
- Abbott DW, Thompson ME, Robinson-Benion C, et al.: BRCA1 expression restores radiation resistance in BRCA1-defective cancer cells through enhancement of transcription-coupled DNA repair. J Biol Chem 274 (26): 18808-12, 1999.
- Abbott DW, Freeman ML, Holt JT: Double-strand break repair deficiency and radiation sensitivity in BRCA2 mutant cancer cells. J Natl Cancer Inst 90 (13): 978-85, 1998.
- Easton DF: Cancer risks in A-T heterozygotes. Int J Radiat Biol 66 (6 Suppl): S177-82, 1994.
- Kleihues P, Schäuble B, zur Hausen A, et al.: Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol 150 (1): 1-13, 1997.
- Pierce LJ, Strawderman M, Narod SA, et al.: Effect of radiotherapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J Clin Oncol 18 (19): 3360-9, 2000.
- Narod SA, Lubinski J, Ghadirian P, et al.: Screening mammography and risk of breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet Oncol 7 (5): 402-6, 2006.
- Andrieu N, Easton DF, Chang-Claude J, et al.: Effect of chest X-rays on the risk of breast cancer among BRCA1/2 mutation carriers in the international BRCA1/2 carrier cohort study: a report from the EMBRACE, GENEPSO, GEO-HEBON, and IBCCS Collaborators' Group. J Clin Oncol 24 (21): 3361-6, 2006.
- Goldfrank D, Chuai S, Bernstein JL, et al.: Effect of mammography on breast cancer risk in women with mutations in BRCA1 or BRCA2. Cancer Epidemiol Biomarkers Prev 15 (11): 2311-3, 2006.
- Gronwald J, Pijpe A, Byrski T, et al.: Early radiation exposures and BRCA1-associated breast cancer in young women from Poland. Breast Cancer Res Treat 112 (3): 581-4, 2008.
- Pijpe A, Andrieu N, Easton DF, et al.: Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK). BMJ 345: e5660, 2012.
- Giannakeas V, Lubinski J, Gronwald J, et al.: Mammography screening and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers: a prospective study. Breast Cancer Res Treat 147 (1): 113-8, 2014.
- Drooger J, Akdeniz D, Pignol JP, et al.: Adjuvant radiotherapy for primary breast cancer in BRCA1 and BRCA2 mutation carriers and risk of contralateral breast cancer with special attention to patients irradiated at younger age. Breast Cancer Res Treat 154 (1): 171-80, 2015.
- Reiner AS, Robson ME, Mellemkjær L, et al.: Radiation Treatment, ATM, BRCA1/2, and CHEK2*1100delC Pathogenic Variants and Risk of Contralateral Breast Cancer. J Natl Cancer Inst 112 (12): 1275-1279, 2020.
- Smith-Warner SA, Spiegelman D, Yaun SS, et al.: Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 279 (7): 535-40, 1998.
- Hamajima N, Hirose K, Tajima K, et al.: Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer 87 (11): 1234-45, 2002.
- McGuire V, John EM, Felberg A, et al.: No increased risk of breast cancer associated with alcohol consumption among carriers of BRCA1 and BRCA2 mutations ages <50 years. Cancer Epidemiol Biomarkers Prev 15 (8): 1565-7, 2006.
- Dennis J, Ghadirian P, Little J, et al.: Alcohol consumption and the risk of breast cancer among BRCA1 and BRCA2 mutation carriers. Breast 19 (6): 479-83, 2010.
- Cybulski C, Lubinski J, Huzarski T, et al.: Prospective evaluation of alcohol consumption and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 151 (2): 435-41, 2015.
- Brunet JS, Ghadirian P, Rebbeck TR, et al.: Effect of smoking on breast cancer in carriers of mutant BRCA1 or BRCA2 genes. J Natl Cancer Inst 90 (10): 761-6, 1998.
- Ghadirian P, Lubinski J, Lynch H, et al.: Smoking and the risk of breast cancer among carriers of BRCA mutations. Int J Cancer 110 (3): 413-6, 2004.
- Li H, Terry MB, Antoniou AC, et al.: Alcohol Consumption, Cigarette Smoking, and Risk of Breast Cancer for BRCA1 and BRCA2 Mutation Carriers: Results from The BRCA1 and BRCA2 Cohort Consortium. Cancer Epidemiol Biomarkers Prev 29 (2): 368-378, 2020.
- Zeinomar N, Knight JA, Genkinger JM, et al.: Alcohol consumption, cigarette smoking, and familial breast cancer risk: findings from the Prospective Family Study Cohort (ProF-SC). Breast Cancer Res 21 (1): 128, 2019.
- Lammert J, Lubinski J, Gronwald J, et al.: Physical activity during adolescence and young adulthood and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 169 (3): 561-571, 2018.
- Kehm RD, Genkinger JM, MacInnis RJ, et al.: Recreational Physical Activity Is Associated with Reduced Breast Cancer Risk in Adult Women at High Risk for Breast Cancer: A Cohort Study of Women Selected for Familial and Genetic Risk. Cancer Res 80 (1): 116-125, 2020.
- Amos CI, Struewing JP: Genetic epidemiology of epithelial ovarian cancer. Cancer 71 (2 Suppl): 566-72, 1993.
- Stratton JF, Pharoah P, Smith SK, et al.: A systematic review and meta-analysis of family history and risk of ovarian cancer. Br J Obstet Gynaecol 105 (5): 493-9, 1998.
- Whittemore AS, Harris R, Itnyre J: Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol 136 (10): 1184-203, 1992.
- Brinton LA, Lamb EJ, Moghissi KS, et al.: Ovarian cancer risk after the use of ovulation-stimulating drugs. Obstet Gynecol 103 (6): 1194-203, 2004.
- Gronwald J, Byrski T, Huzarski T, et al.: Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland. Breast Cancer Res Treat 95 (2): 105-9, 2006.
- McLaughlin JR, Risch HA, Lubinski J, et al.: Reproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet Oncol 8 (1): 26-34, 2007.
- Antoniou AC, Rookus M, Andrieu N, et al.: Reproductive and hormonal factors, and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers: results from the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev 18 (2): 601-10, 2009.
- Narod SA, Sun P, Ghadirian P, et al.: Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet 357 (9267): 1467-70, 2001.
- Kotsopoulos J, Lubinski J, Gronwald J, et al.: Factors influencing ovulation and the risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. Int J Cancer 137 (5): 1136-46, 2015.
- Rodriguez C, Patel AV, Calle EE, et al.: Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA 285 (11): 1460-5, 2001.
- Riman T, Dickman PW, Nilsson S, et al.: Hormone replacement therapy and the risk of invasive epithelial ovarian cancer in Swedish women. J Natl Cancer Inst 94 (7): 497-504, 2002.
- Lacey JV, Mink PJ, Lubin JH, et al.: Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA 288 (3): 334-41, 2002.
- Anderson GL, Judd HL, Kaunitz AM, et al.: Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. JAMA 290 (13): 1739-48, 2003.
- Tortolero-Luna G, Mitchell MF: The epidemiology of ovarian cancer. J Cell Biochem Suppl 23: 200-7, 1995.
- Hankinson SE, Hunter DJ, Colditz GA, et al.: Tubal ligation, hysterectomy, and risk of ovarian cancer. A prospective study. JAMA 270 (23): 2813-8, 1993.
- Rutter JL, Wacholder S, Chetrit A, et al.: Gynecologic surgeries and risk of ovarian cancer in women with BRCA1 and BRCA2 Ashkenazi founder mutations: an Israeli population-based case-control study. J Natl Cancer Inst 95 (14): 1072-8, 2003.
- Kauff ND, Satagopan JM, Robson ME, et al.: Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 346 (21): 1609-15, 2002.
- Rebbeck TR, Lynch HT, Neuhausen SL, et al.: Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 346 (21): 1616-22, 2002.
- Kotsopoulos J, Huzarski T, Gronwald J, et al.: Bilateral Oophorectomy and Breast Cancer Risk in BRCA1 and BRCA2 Mutation Carriers. J Natl Cancer Inst 109 (1): , 2017.
- John EM, Whittemore AS, Harris R, et al.: Characteristics relating to ovarian cancer risk: collaborative analysis of seven U.S. case-control studies. Epithelial ovarian cancer in black women. Collaborative Ovarian Cancer Group. J Natl Cancer Inst 85 (2): 142-7, 1993.
- Narod SA, Risch H, Moslehi R, et al.: Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med 339 (7): 424-8, 1998.
- Whittemore AS, Balise RR, Pharoah PD, et al.: Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer 91 (11): 1911-5, 2004.
- McGuire V, Felberg A, Mills M, et al.: Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol 160 (7): 613-8, 2004.
- Modan B, Hartge P, Hirsh-Yechezkel G, et al.: Parity, oral contraceptives, and the risk of ovarian cancer among carriers and noncarriers of a BRCA1 or BRCA2 mutation. N Engl J Med 345 (4): 235-40, 2001.
- Soliman PT, Oh JC, Schmeler KM, et al.: Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol 105 (3): 575-80, 2005.
- Vasen HF, Stormorken A, Menko FH, et al.: MSH2 mutation carriers are at higher risk of cancer than MLH1 mutation carriers: a study of hereditary nonpolyposis colorectal cancer families. J Clin Oncol 19 (20): 4074-80, 2001.
- Daniels MS: Genetic testing by cancer site: uterus. Cancer J 18 (4): 338-42, 2012 Jul-Aug.
- Dunlop MG, Farrington SM, Nicholl I, et al.: Population carrier frequency of hMSH2 and hMLH1 mutations. Br J Cancer 83 (12): 1643-5, 2000.
- de la Chapelle A: The incidence of Lynch syndrome. Fam Cancer 4 (3): 233-7, 2005.
- Ring KL, Bruegl AS, Allen BA, et al.: Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol 29 (11): 1381-1389, 2016.
- Shu CA, Pike MC, Jotwani AR, et al.: Uterine Cancer After Risk-Reducing Salpingo-oophorectomy Without Hysterectomy in Women With BRCA Mutations. JAMA Oncol 2 (11): 1434-1440, 2016.
- de Jonge MM, de Kroon CD, Jenner DJ, et al.: Endometrial Cancer Risk in Women With Germline BRCA1 or BRCA2 Mutations: Multicenter Cohort Study. J Natl Cancer Inst 113 (9): 1203-1211, 2021.
- McPherson CP, Sellers TA, Potter JD, et al.: Reproductive factors and risk of endometrial cancer. The Iowa Women's Health Study. Am J Epidemiol 143 (12): 1195-202, 1996.
- Dossus L, Allen N, Kaaks R, et al.: Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 127 (2): 442-51, 2010.
- Shapiro S, Kelly JP, Rosenberg L, et al.: Risk of localized and widespread endometrial cancer in relation to recent and discontinued use of conjugated estrogens. N Engl J Med 313 (16): 969-72, 1985.
- Ziel HK, Finkle WD: Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med 293 (23): 1167-70, 1975.
- Fisher B, Costantino JP, Redmond CK, et al.: Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 86 (7): 527-37, 1994.
- Pike MC, Peters RK, Cozen W, et al.: Estrogen-progestin replacement therapy and endometrial cancer. J Natl Cancer Inst 89 (15): 1110-6, 1997.
- Fournier A, Dossus L, Mesrine S, et al.: Risks of endometrial cancer associated with different hormone replacement therapies in the E3N cohort, 1992-2008. Am J Epidemiol 180 (5): 508-17, 2014.
- Weiss NS, Sayvetz TA: Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med 302 (10): 551-4, 1980.
- Soini T, Hurskainen R, Grénman S, et al.: Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol 124 (2 Pt 1): 292-9, 2014.
- Lindor NM, McMaster ML, Lindor CJ, et al.: Concise handbook of familial cancer susceptibility syndromes - second edition. J Natl Cancer Inst Monogr (38): 1-93, 2008.
- Vasen HF, Offerhaus GJ, den Hartog Jager FC, et al.: The tumour spectrum in hereditary non-polyposis colorectal cancer: a study of 24 kindreds in the Netherlands. Int J Cancer 46 (1): 31-4, 1990.
- Watson P, Lynch HT: Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer 71 (3): 677-85, 1993.
- Watson P, Vasen HF, Mecklin JP, et al.: The risk of endometrial cancer in hereditary nonpolyposis colorectal cancer. Am J Med 96 (6): 516-20, 1994.
- Aarnio M, Mecklin JP, Aaltonen LA, et al.: Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer 64 (6): 430-3, 1995.
- Raymond VM, Everett JN, Furtado LV, et al.: Adrenocortical carcinoma is a lynch syndrome-associated cancer. J Clin Oncol 31 (24): 3012-8, 2013.
- Raymond VM, Mukherjee B, Wang F, et al.: Elevated risk of prostate cancer among men with Lynch syndrome. J Clin Oncol 31 (14): 1713-8, 2013.
- Suspiro A, Fidalgo P, Cravo M, et al.: The Muir-Torre syndrome: a rare variant of hereditary nonpolyposis colorectal cancer associated with hMSH2 mutation. Am J Gastroenterol 93 (9): 1572-4, 1998.
- Kerber RA, Slattery ML: Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol 146 (3): 244-8, 1997.
- Parent ME, Ghadirian P, Lacroix A, et al.: The reliability of recollections of family history: implications for the medical provider. J Cancer Educ 12 (2): 114-20, 1997 Summer.
- Ready K, Litton JK, Arun BK: Clinical application of breast cancer risk assessment models. Future Oncol 6 (3): 355-65, 2010.
- Amir E, Freedman OC, Seruga B, et al.: Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst 102 (10): 680-91, 2010.
- Rosner BA, Colditz GA, Webb PM, et al.: Mathematical models of ovarian cancer incidence. Epidemiology 16 (4): 508-15, 2005.
- Pfeiffer RM, Park Y, Kreimer AR, et al.: Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS Med 10 (7): e1001492, 2013.
- Quante AS, Whittemore AS, Shriver T, et al.: Practical problems with clinical guidelines for breast cancer prevention based on remaining lifetime risk. J Natl Cancer Inst 107 (7): , 2015.
- MacInnis RJ, Knight JA, Chung WK, et al.: Comparing 5-Year and Lifetime Risks of Breast Cancer using the Prospective Family Study Cohort. J Natl Cancer Inst 113 (6): 785-791, 2021.
- Gail MH, Mai PL: Comparing breast cancer risk assessment models. J Natl Cancer Inst 102 (10): 665-8, 2010.
- Quante AS, Whittemore AS, Shriver T, et al.: Breast cancer risk assessment across the risk continuum: genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res 14 (6): R144, 2012.
- Gail MH, Brinton LA, Byar DP, et al.: Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 81 (24): 1879-86, 1989.
- Colditz GA, Rosner B: Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses' Health Study. Am J Epidemiol 152 (10): 950-64, 2000.
- Claus EB, Risch N, Thompson WD: Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 73 (3): 643-51, 1994.
- Tyrer J, Duffy SW, Cuzick J: A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23 (7): 1111-30, 2004.
- Parmigiani G, Berry D, Aguilar O: Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet 62 (1): 145-58, 1998.
- Antoniou AC, Pharoah PD, McMullan G, et al.: A comprehensive model for familial breast cancer incorporating BRCA1, BRCA2 and other genes. Br J Cancer 86 (1): 76-83, 2002.
- Antoniou AC, Pharoah PP, Smith P, et al.: The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer 91 (8): 1580-90, 2004.
- Antoniou AC, Cunningham AP, Peto J, et al.: The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer 98 (8): 1457-66, 2008.
- Mavaddat N, Ficorella L, Carver T, et al.: Incorporating Alternative Polygenic Risk Scores into the BOADICEA Breast Cancer Risk Prediction Model. Cancer Epidemiol Biomarkers Prev 32 (3): 422-427, 2023.
- Carver T, Hartley S, Lee A, et al.: CanRisk Tool-A Web Interface for the Prediction of Breast and Ovarian Cancer Risk and the Likelihood of Carrying Genetic Pathogenic Variants. Cancer Epidemiol Biomarkers Prev 30 (3): 469-473, 2021.
- Anothaisintawee T, Teerawattananon Y, Wiratkapun C, et al.: Risk prediction models of breast cancer: a systematic review of model performances. Breast Cancer Res Treat 133 (1): 1-10, 2012.
- Amir E, Evans DG, Shenton A, et al.: Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet 40 (11): 807-14, 2003.
- Laitman Y, Simeonov M, Keinan-Boker L, et al.: Breast cancer risk prediction accuracy in Jewish Israeli high-risk women using the BOADICEA and IBIS risk models. Genet Res (Camb) 95 (6): 174-7, 2013.
- MacInnis RJ, Bickerstaffe A, Apicella C, et al.: Prospective validation of the breast cancer risk prediction model BOADICEA and a batch-mode version BOADICEACentre. Br J Cancer 109 (5): 1296-301, 2013.
- Vilmun BM, Vejborg I, Lynge E, et al.: Impact of adding breast density to breast cancer risk models: A systematic review. Eur J Radiol 127: 109019, 2020.
- Terry MB, Liao Y, Whittemore AS, et al.: 10-year performance of four models of breast cancer risk: a validation study. Lancet Oncol 20 (4): 504-517, 2019.
- Claus EB, Risch N, Thompson WD: The calculation of breast cancer risk for women with a first degree family history of ovarian cancer. Breast Cancer Res Treat 28 (2): 115-20, 1993.
- Bondy ML, Lustbader ED, Halabi S, et al.: Validation of a breast cancer risk assessment model in women with a positive family history. J Natl Cancer Inst 86 (8): 620-5, 1994.
- Spiegelman D, Colditz GA, Hunter D, et al.: Validation of the Gail et al. model for predicting individual breast cancer risk. J Natl Cancer Inst 86 (8): 600-7, 1994.
- Rockhill B, Spiegelman D, Byrne C, et al.: Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst 93 (5): 358-66, 2001.
- Costantino JP, Gail MH, Pee D, et al.: Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst 91 (18): 1541-8, 1999.
- Bondy ML, Newman LA: Breast cancer risk assessment models: applicability to African-American women. Cancer 97 (1 Suppl): 230-5, 2003.
- Schonfeld SJ, Pee D, Greenlee RT, et al.: Effect of changing breast cancer incidence rates on the calibration of the Gail model. J Clin Oncol 28 (14): 2411-7, 2010.
- Gail MH, Costantino JP, Pee D, et al.: Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst 99 (23): 1782-92, 2007.
- Gail MH: Discriminatory accuracy from single-nucleotide polymorphisms in models to predict breast cancer risk. J Natl Cancer Inst 100 (14): 1037-41, 2008.
- Gail MH: Value of adding single-nucleotide polymorphism genotypes to a breast cancer risk model. J Natl Cancer Inst 101 (13): 959-63, 2009.
- Barlow WE, White E, Ballard-Barbash R, et al.: Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst 98 (17): 1204-14, 2006.
- Tice JA, Cummings SR, Ziv E, et al.: Mammographic breast density and the Gail model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat 94 (2): 115-22, 2005.
- Lee AJ, Cunningham AP, Tischkowitz M, et al.: Incorporating truncating variants in PALB2, CHEK2, and ATM into the BOADICEA breast cancer risk model. Genet Med 18 (12): 1190-1198, 2016.
- MacInnis RJ, Liao Y, Knight JA, et al.: Considerations When Using Breast Cancer Risk Models for Women with Negative BRCA1/BRCA2 Mutation Results. J Natl Cancer Inst 112 (4): 418-422, 2020.
- Liang X, Harris HR, Hendryx M, et al.: Sleep Characteristics and Risk of Ovarian Cancer Among Postmenopausal Women. Cancer Prev Res (Phila) 14 (1): 55-64, 2021.
- Jiang Y, Wang C, Zhou S: Artificial intelligence-based risk stratification, accurate diagnosis and treatment prediction in gynecologic oncology. Semin Cancer Biol 96: 82-99, 2023.
- Shi J, Kraft P, Rosner BA, et al.: Risk prediction models for endometrial cancer: development and validation in an international consortium. J Natl Cancer Inst 115 (5): 552-559, 2023.
- Barnetson RA, Tenesa A, Farrington SM, et al.: Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med 354 (26): 2751-63, 2006.
- Kastrinos F, Uno H, Ukaegbu C, et al.: Development and Validation of the PREMM5 Model for Comprehensive Risk Assessment of Lynch Syndrome. J Clin Oncol 35 (19): 2165-2172, 2017.
- Chen S, Wang W, Lee S, et al.: Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA 296 (12): 1479-87, 2006.
- Khan O, Blanco A, Conrad P, et al.: Performance of Lynch syndrome predictive models in a multi-center US referral population. Am J Gastroenterol 106 (10): 1822-7; quiz 1828, 2011.
- Mercado RC, Hampel H, Kastrinos F, et al.: Performance of PREMM(1,2,6), MMRpredict, and MMRpro in detecting Lynch syndrome among endometrial cancer cases. Genet Med 14 (7): 670-80, 2012.
- Shazly SA, Coronado PJ, Yılmaz E, et al.: Endometrial Cancer Individualized Scoring System (ECISS): A machine learning-based prediction model of endometrial cancer prognosis. Int J Gynaecol Obstet 161 (3): 760-768, 2023.
- Couch FJ, DeShano ML, Blackwood MA, et al.: BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 336 (20): 1409-15, 1997.
- Shattuck-Eidens D, Oliphant A, McClure M, et al.: BRCA1 sequence analysis in women at high risk for susceptibility mutations. Risk factor analysis and implications for genetic testing. JAMA 278 (15): 1242-50, 1997.
- Frank TS, Manley SA, Olopade OI, et al.: Sequence analysis of BRCA1 and BRCA2: correlation of mutations with family history and ovarian cancer risk. J Clin Oncol 16 (7): 2417-25, 1998.
- Frank TS, Deffenbaugh AM, Reid JE, et al.: Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 20 (6): 1480-90, 2002.
- Evans DG, Eccles DM, Rahman N, et al.: A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet 41 (6): 474-80, 2004.
- Apicella C, Dowty JG, Dite GS, et al.: Validation study of the LAMBDA model for predicting the BRCA1 or BRCA2 mutation carrier status of North American Ashkenazi Jewish women. Clin Genet 72 (2): 87-97, 2007.
- Risch HA, McLaughlin JR, Cole DE, et al.: Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet 68 (3): 700-10, 2001.
- Malone KE, Daling JR, Doody DR, et al.: Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res 66 (16): 8297-308, 2006.
- Struewing JP, Abeliovich D, Peretz T, et al.: The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet 11 (2): 198-200, 1995.
- Oddoux C, Struewing JP, Clayton CM, et al.: The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet 14 (2): 188-90, 1996.
- Warner E, Foulkes W, Goodwin P, et al.: Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 91 (14): 1241-7, 1999.
- Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer 83 (10): 1301-8, 2000.
- Euhus DM, Smith KC, Robinson L, et al.: Pretest prediction of BRCA1 or BRCA2 mutation by risk counselors and the computer model BRCAPRO. J Natl Cancer Inst 94 (11): 844-51, 2002.
- de la Hoya M, Díez O, Pérez-Segura P, et al.: Pre-test prediction models of BRCA1 or BRCA2 mutation in breast/ovarian families attending familial cancer clinics. J Med Genet 40 (7): 503-10, 2003.
- Katki HA: Incorporating medical interventions into carrier probability estimation for genetic counseling. BMC Med Genet 8: 13, 2007.
- Weitzel JN, Lagos VI, Cullinane CA, et al.: Limited family structure and BRCA gene mutation status in single cases of breast cancer. JAMA 297 (23): 2587-95, 2007.
- Jervis S, Song H, Lee A, et al.: A risk prediction algorithm for ovarian cancer incorporating BRCA1, BRCA2, common alleles and other familial effects. J Med Genet 52 (7): 465-75, 2015.
- Oros KK, Ghadirian P, Maugard CM, et al.: Application of BRCA1 and BRCA2 mutation carrier prediction models in breast and/or ovarian cancer families of French Canadian descent. Clin Genet 70 (4): 320-9, 2006.
- Vogel KJ, Atchley DP, Erlichman J, et al.: BRCA1 and BRCA2 genetic testing in Hispanic patients: mutation prevalence and evaluation of the BRCAPRO risk assessment model. J Clin Oncol 25 (29): 4635-41, 2007.
- Kurian AW, Gong GD, John EM, et al.: Performance of prediction models for BRCA mutation carriage in three racial/ethnic groups: findings from the Northern California Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev 18 (4): 1084-91, 2009.
- Kurian AW, Gong GD, Chun NM, et al.: Performance of BRCA1/2 mutation prediction models in Asian Americans. J Clin Oncol 26 (29): 4752-8, 2008.
- Berry DA, Parmigiani G, Sanchez J, et al.: Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. J Natl Cancer Inst 89 (3): 227-38, 1997.
- Barcenas CH, Hosain GM, Arun B, et al.: Assessing BRCA carrier probabilities in extended families. J Clin Oncol 24 (3): 354-60, 2006.
- Kang HH, Williams R, Leary J, et al.: Evaluation of models to predict BRCA germline mutations. Br J Cancer 95 (7): 914-20, 2006.
- Antoniou AC, Hardy R, Walker L, et al.: Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet 45 (7): 425-31, 2008.
- Fischer C, Kuchenbäcker K, Engel C, et al.: Evaluating the performance of the breast cancer genetic risk models BOADICEA, IBIS, BRCAPRO and Claus for predicting BRCA1/2 mutation carrier probabilities: a study based on 7352 families from the German Hereditary Breast and Ovarian Cancer Consortium. J Med Genet 50 (6): 360-7, 2013.
- Biswas S, Tankhiwale N, Blackford A, et al.: Assessing the added value of breast tumor markers in genetic risk prediction model BRCAPRO. Breast Cancer Res Treat 133 (1): 347-55, 2012.
- Tai YC, Chen S, Parmigiani G, et al.: Incorporating tumor immunohistochemical markers in BRCA1 and BRCA2 carrier prediction. Breast Cancer Res 10 (2): 401, 2008.
- Ready KJ, Vogel KJ, Atchley DP, et al.: Accuracy of the BRCAPRO model among women with bilateral breast cancer. Cancer 115 (4): 725-30, 2009.
- Huo D, Senie RT, Daly M, et al.: Prediction of BRCA Mutations Using the BRCAPRO Model in Clinic-Based African American, Hispanic, and Other Minority Families in the United States. J Clin Oncol 27 (8): 1184-90, 2009.
- Daniels MS, Babb SA, King RH, et al.: Underestimation of risk of a BRCA1 or BRCA2 mutation in women with high-grade serous ovarian cancer by BRCAPRO: a multi-institution study. J Clin Oncol 32 (12): 1249-55, 2014.
- Robson ME, Storm CD, Weitzel J, et al.: American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol 28 (5): 893-901, 2010.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023.
Available online with free registration. Last accessed September 18, 2024. - Statement of the American Society of Human Genetics on genetic testing for breast and ovarian cancer predisposition. Am J Hum Genet 55 (5): i-iv, 1994.
- Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015.
- Owens DK, Davidson KW, Krist AH, et al.: Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 322 (7): 652-665, 2019.
- Lancaster JM, Powell CB, Kauff ND, et al.: Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 107 (2): 159-62, 2007.
- Manahan ER, Kuerer HM, Sebastian M, et al.: Consensus Guidelines on Genetic` Testing for Hereditary Breast Cancer from the American Society of Breast Surgeons. Ann Surg Oncol 26 (10): 3025-3031, 2019.
- Beitsch PD, Whitworth PW, Hughes K, et al.: Underdiagnosis of Hereditary Breast Cancer: Are Genetic Testing Guidelines a Tool or an Obstacle? J Clin Oncol 37 (6): 453-460, 2019.
- Cropper C, Woodson A, Arun B, et al.: Evaluating the NCCN Clinical Criteria for Recommending BRCA1 and BRCA2 Genetic Testing in Patients With Breast Cancer. J Natl Compr Canc Netw 15 (6): 797-803, 2017.
- Grindedal EM, Heramb C, Karsrud I, et al.: Current guidelines for BRCA testing of breast cancer patients are insufficient to detect all mutation carriers. BMC Cancer 17 (1): 438, 2017.
- Yadav S, Hu C, Hart SN, et al.: Evaluation of Germline Genetic Testing Criteria in a Hospital-Based Series of Women With Breast Cancer. J Clin Oncol 38 (13): 1409-1418, 2020.
- Kurian AW, Bernhisel R, Larson K, et al.: Prevalence of Pathogenic Variants in Cancer Susceptibility Genes Among Women With Postmenopausal Breast Cancer. JAMA 323 (10): 995-997, 2020.
- Couch FJ, Nathanson KL, Offit K: Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science 343 (6178): 1466-70, 2014.
- Theobald KA, Susswein LR, Marshall ML, et al.: Utility of Expedited Hereditary Cancer Testing in the Surgical Management of Patients with a New Breast Cancer Diagnosis. Ann Surg Oncol 25 (12): 3556-3562, 2018.
- Gornick MC, Kurian AW, An LC, et al.: Knowledge regarding and patterns of genetic testing in patients newly diagnosed with breast cancer participating in the iCanDecide trial. Cancer 124 (20): 4000-4009, 2018.
- Chiba A, Hoskin TL, Hallberg EJ, et al.: Impact that Timing of Genetic Mutation Diagnosis has on Surgical Decision Making and Outcome for BRCA1/BRCA2 Mutation Carriers with Breast Cancer. Ann Surg Oncol 23 (10): 3232-8, 2016.
- Obermair A, Youlden DR, Baade PD, et al.: The impact of risk-reducing hysterectomy and bilateral salpingo-oophorectomy on survival in patients with a history of breast cancer--a population-based data linkage study. Int J Cancer 134 (9): 2211-22, 2014.
- Byrski T, Dent R, Blecharz P, et al.: Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res 14 (4): R110, 2012.
- Robson M, Im SA, Senkus E, et al.: Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 377 (6): 523-533, 2017.
- Heymann S, Delaloge S, Rahal A, et al.: Radio-induced malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndrome. Radiat Oncol 5: 104, 2010.
- Bougeard G, Renaux-Petel M, Flaman JM, et al.: Revisiting Li-Fraumeni Syndrome From TP53 Mutation Carriers. J Clin Oncol 33 (21): 2345-52, 2015.
- Rebbeck TR, Kauff ND, Domchek SM: Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst 101 (2): 80-7, 2009.
- Kotsopoulos J, Lubinski J, Lynch HT, et al.: Oophorectomy after menopause and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev 21 (7): 1089-96, 2012.
- Cass I, Baldwin RL, Varkey T, et al.: Improved survival in women with BRCA-associated ovarian carcinoma. Cancer 97 (9): 2187-95, 2003.
- Tan DS, Rothermundt C, Thomas K, et al.: "BRCAness" syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol 26 (34): 5530-6, 2008.
- Moore K, Colombo N, Scambia G, et al.: Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 379 (26): 2495-2505, 2018.
- Practice Bulletin No. 149: Endometrial cancer. Obstet Gynecol 125 (4): 1006-26, 2015.
- Ott PA, Bang YJ, Berton-Rigaud D, et al.: Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J Clin Oncol 35 (22): 2535-2541, 2017.
- Walsh T, Casadei S, Lee MK, et al.: Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A 108 (44): 18032-7, 2011.
- Frey MK, Kim SH, Bassett RY, et al.: Rescreening for genetic mutations using multi-gene panel testing in patients who previously underwent non-informative genetic screening. Gynecol Oncol 139 (2): 211-5, 2015.
- Desmond A, Kurian AW, Gabree M, et al.: Clinical Actionability of Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Risk Assessment. JAMA Oncol 1 (7): 943-51, 2015.
- Couch FJ, Shimelis H, Hu C, et al.: Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol 3 (9): 1190-1196, 2017.
- Weitzel JN, Neuhausen SL, Adamson A, et al.: Pathogenic and likely pathogenic variants in PALB2, CHEK2, and other known breast cancer susceptibility genes among 1054 BRCA-negative Hispanics with breast cancer. Cancer 125 (16): 2829-2836, 2019.
- Frey MK, Kopparam RV, Ni Zhou Z, et al.: Prevalence of nonfounder BRCA1/2 mutations in Ashkenazi Jewish patients presenting for genetic testing at a hereditary breast and ovarian cancer center. Cancer 125 (5): 690-697, 2019.
- Eoh KJ, Kim JE, Park HS, et al.: Detection of Germline Mutations in Patients with Epithelial Ovarian Cancer Using Multi-gene Panels: Beyond BRCA1/2. Cancer Res Treat 50 (3): 917-925, 2018.
- Tung N, Lin NU, Kidd J, et al.: Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients With Breast Cancer. J Clin Oncol 34 (13): 1460-8, 2016.
- Buys SS, Sandbach JF, Gammon A, et al.: A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer 123 (10): 1721-1730, 2017.
- Pritzlaff M, Summerour P, McFarland R, et al.: Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat 161 (3): 575-586, 2017.
- Yadav S, Reeves A, Campian S, et al.: Outcomes of retesting BRCA negative patients using multigene panels. Fam Cancer 16 (3): 319-328, 2017.
- Crawford B, Adams SB, Sittler T, et al.: Multi-gene panel testing for hereditary cancer predisposition in unsolved high-risk breast and ovarian cancer patients. Breast Cancer Res Treat 163 (2): 383-390, 2017.
- Kurian AW, Ward KC, Howlader N, et al.: Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J Clin Oncol 37 (15): 1305-1315, 2019.
- Carter NJ, Marshall ML, Susswein LR, et al.: Germline pathogenic variants identified in women with ovarian tumors. Gynecol Oncol 151 (3): 481-488, 2018.
- Dorling L, Carvalho S, Allen J, et al.: Breast Cancer Risk Genes - Association Analysis in More than 113,000 Women. N Engl J Med 384 (5): 428-439, 2021.
- Hu C, Hart SN, Gnanaolivu R, et al.: A Population-Based Study of Genes Previously Implicated in Breast Cancer. N Engl J Med 384 (5): 440-451, 2021.
- Shimelis H, LaDuca H, Hu C, et al.: Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. J Natl Cancer Inst 110 (8): 855-862, 2018.
- Palmer JR, Polley EC, Hu C, et al.: Contribution of Germline Predisposition Gene Mutations to Breast Cancer Risk in African American Women. J Natl Cancer Inst 112 (12): 1213-1221, 2020.
- Díaz-Zabala H, Guo X, Ping J, et al.: Evaluating breast cancer predisposition genes in women of African ancestry. Genet Med 24 (7): 1468-1475, 2022.
- Southey MC, Goldgar DE, Winqvist R, et al.: PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS. J Med Genet 53 (12): 800-811, 2016.
- Kurian AW, Ward KC, Hamilton AS, et al.: Uptake, Results, and Outcomes of Germline Multiple-Gene Sequencing After Diagnosis of Breast Cancer. JAMA Oncol 4 (8): 1066-1072, 2018.
Penetrance of Inherited Susceptibility to Hereditary Breast and / or Gynecologic Cancers
The proportion of individuals carrying a pathogenic variant who will manifest a certain disease is referred to as penetrance. In general, common genetic variants that are associated with cancer susceptibility have a lower penetrance than rare genetic variants. This is depicted in
Figure 4. Genetic architecture of cancer risk. This graph depicts the general finding of a low relative risk associated with common, low-penetrance genetic variants, such as single-nucleotide polymorphisms identified in genome-wide association studies, and a higher relative risk associated with rare, high-penetrance genetic variants, such as pathogenic variants in the BRCA1/BRCA2 genes associated with hereditary breast and ovarian cancer and the mismatch repair genes associated with Lynch syndrome.
Throughout this summary, we discuss studies that report on relative and absolute risks (ARs). These are two important but different concepts. Relative risk (RR) refers to an estimate of risk relative to another group (e.g., risk of an outcome like breast cancer for women who are exposed to a risk factor relative to the risk of breast cancer for women who are unexposed to the same risk factor). RR measures that are greater than 1 mean that the risk for those captured in the numerator (i.e., the exposed) is higher than the risk for those captured in the denominator (i.e., the unexposed). RR measures that are less than 1 mean that the risk for those captured in the numerator (i.e., the exposed) is lower than the risk for those captured in the denominator (i.e., the unexposed). Measures with similar relative interpretations include the odds ratio (OR), hazard ratio, and risk ratio.
AR measures consider the number of people who have a particular outcome, the number of people in a population who could have the outcome, and person-time (the period of time during which an individual was at risk of having the outcome). AR measures also reflect the absolute burden of an outcome in a population. Absolute measures include risks and rates and can be expressed over a specific time frame (e.g., 1 year, 5 years) or overall lifetime. Cumulative risk is a measure of risk that occurs over a defined time period. For example, overall lifetime risk is a type of cumulative risk that is usually calculated on the basis of a given life expectancy (e.g., 80 or 90 years). Cumulative risk can also be presented over other time frames (e.g., up to age 50 years).
Large RR measures do not mean that there will be large effects in the actual number of individuals at a population level because the disease outcome may be quite rare. For example, the RR for smoking is much higher for lung cancer than for heart disease, but the absolute difference between smokers and nonsmokers is greater for heart disease, the more-common outcome, than for lung cancer, the more-rare outcome.
Therefore, in evaluating the effect of exposures and biological markers on disease prevention across the continuum, it is important to recognize the differences between relative and absolute effects in weighing the overall impact of a given risk factor. For example, the magnitude is in the range of 30% (e.g., ORs or RRs of 1.3) for many breast cancer risk factors, which means that women with a risk factor (e.g., alcohol consumption, late age at first birth, oral contraceptive use, postmenopausal body size) have a 30% relative increase in breast cancer in comparison with what they would have if they did not have that risk factor. But the absolute increase in risk is based on the underlying AR of disease.
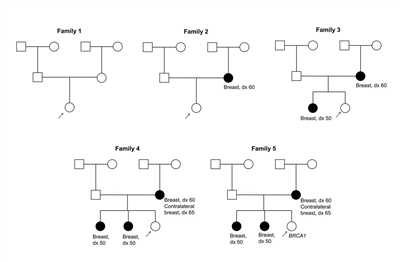
Figure 5. These five pedigrees depict probands with varying degrees of family history. Table 2 accompanies this figure.
| Family History | Lifetime Risk (%) | Lifetime Risk After Risk Factor Modification (%) | Absolute Risk Difference (%) | Relative Risk |
|---|---|---|---|---|
| a Refer to |
||||
| Low (Family 1) | 10.9 | 8.4 | 2.50 | 1.29 (29% increased risk) |
| Moderate (Family 2) | 21.6 | 16.8 | 4.80 | 1.28 (28% increased risk) |
| Moderate/high (Family 3) | 27.1 | 21.3 | 5.80 | 1.27 (27% increased risk) |
| High (Family 4) | 32.0 | 25.3 | 6.70 | 1.26 (26% increased risk) |
| BRCA1pathogenic variant (Family 5) | 53.7 | 44.2 | 9.50 | 1.21 (21% increased risk) |
With the increasing use of multigene panel tests, a framework for cancer risk management among individuals with pathogenic variants detected in novel genes has been described [
References:
- Quante AS, Herz J, Whittemore AS, et al.: Assessing absolute changes in breast cancer risk due to modifiable risk factors. Breast Cancer Res Treat 152 (1): 193-7, 2015.
- Tung N, Domchek SM, Stadler Z, et al.: Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol 13 (9): 581-8, 2016.
Genes Associated With Breast and / or Gynecologic Cancer Susceptibility
Several genes are found to be associated with the development of breast and/or gynecologic cancers. These genes are categorized as high-penetrance, moderate-penetrance, and low-penetrance in this summary. The high- and moderate-penetrance genes are summarized in
| Cancer Susceptibilitya | Moderate-Penetrance Genesb | High-Penetrance Genes |
|---|---|---|
| a Other cancers may be associated with the genes in this table. | ||
| b Other genes discussed in the |
||
| Breast cancer | ATM,BRIP1,CHEK2,FANCD2,RAD51C | BRCA1,BRCA2,CDH1,PALB2,PTEN,STK11,TP53 |
| Ovarian cancer | ATM,BRIP1,EPCAM,MLH1,MSH2,MSH6,RAD51C | BRCA1,BRCA2 |
| Endometrial cancer | EPCAM,MLH1,MSH2,MSH6,PMS2,PTEN | |
High-Penetrance Breast and / or Gynecologic Cancer Susceptibility Genes
BRCA1andBRCA2
Pathogenic variants in the BRCA1 and BRCA2 genes are associated with increased risks of breast, ovarian, prostate, pancreatic, and other cancers. For more information about BRCA1 and BRCA2 pathogenic variants and BRCA-associated cancer risks, see
Lynch Syndrome
Lynch syndrome is characterized by autosomal dominant inheritance of susceptibility to predominantly right-sided colon cancer, endometrial cancer, ovarian cancer, and other extracolonic cancers (including cancer of the renal pelvis, ureter, small bowel, and pancreas), multiple primary cancers, and a young age of onset of cancer.[
After colorectal cancer, endometrial cancer is the second hallmark cancer of a family with Lynch syndrome. Even in the original Family G, described by Dr. Aldred Scott Warthin, numerous family members were noted to have extracolonic cancers including endometrial cancer. Although the first version of the Amsterdam criteria did not include endometrial cancer,[
The lifetime risk of ovarian carcinoma in females with Lynch syndrome is estimated to be as high as 12%, and the reported relative risk (RR) of ovarian cancer has ranged from 3.6 to 13, based on families ascertained from high-risk clinics with known or suspected Lynch syndrome.[
The issue of breast cancer risk in Lynch syndrome has been controversial.
Retrospective studies have been inconsistent, but several have demonstrated microsatellite instability in a proportion of breast cancers from individuals with Lynch syndrome;[
A number of subsequent studies have suggested the presence of higher breast cancer risks than previously published,[
Li-Fraumeni Syndrome (LFS)
Breast cancer is also a component of the rare LFS, in which germline variants of the TP53 gene on chromosome 17p have been documented. Located on chromosome 17p, TP53 encodes a 53kd nuclear phosphoprotein that binds DNA sequences and functions as a negative regulator of cell growth and proliferation in the setting of DNA damage. It is also an active component of programmed cell death.[
LFS is characterized by premenopausal breast cancer in combination with childhood sarcoma, brain tumors, leukemia, and adrenocortical carcinoma.[
Germline variants in TP53 are thought to account for fewer than 1% of breast cancer cases.[
Historical criteria for defining LFS
The term LFS was used for the first time in 1982,[
- Sarcoma before age 45 years;
- A first-degree relative (FDR) with cancer before age 45 years; AND
- Another close relative (FDR or second-degree relative [SDR]) with either cancer before age 45 years or a sarcoma at any age.
Subsequently in 2001, Chompret et al. [
- A proband affected by a narrow-spectrum tumor before age 36 years AND at least one FDR or SDR affected by a narrow-spectrum tumor (other than breast cancer if the proband is affected by breast cancer) before age 46 years or multiple primary tumors; OR
- A proband with multiple primary tumors, two of which belong to the narrow spectrum and the first of which occurred before age 36 years, irrespective of family history; OR
- A proband with adrenocortical carcinoma irrespective of the age at onset and family history.
These criteria were revised in 2009 [
- A proband with a tumor belonging to the LFS tumor spectrum* before age 46 years AND at least one FDR or SDR with an LFS tumor (except breast cancer if proband has breast cancer) before age 56 years or with multiple tumors; OR
- A proband with multiple tumors (except multiple breast tumors), two of which belong to the LFS tumor spectrum and the first of which occurred before age 46 years; OR
- A patient with adrenocortical carcinoma or choroid plexus, irrespective of family history.
*The 2009 Chompret criteria defined the LFS tumor spectrum as including the following cancers: soft tissue sarcoma, osteosarcoma, brain tumor, premenopausal breast cancer, adrenocortical carcinoma, leukemia, and lung bronchoalveolar cancer.
In 2015, Bougeard et al. [
- A proband with a tumor belonging to the LFS tumor spectrum** before age 46 years AND at least one FDR or SDR with LFS tumor (except breast cancer if proband has breast cancer) before age 56 years or with multiple tumors; OR
- A proband with multiple tumors (except multiple breast tumors), two of which belong to the LFS tumor spectrum and the first of which occurred before age 46 years; OR
- A patient with adrenocortical carcinoma, choroid plexus tumor, or rhabdomyosarcoma of embryonal anaplastic subtype, irrespective of family history; OR
- Breast cancer before age 31 years.
**The 2015 Chompret criteria defined the LFS tumor spectrum as including the following cancers: premenopausal breast cancer, soft tissue sarcoma, osteosarcoma, central nervous system (CNS) tumor, and adrenocortical carcinoma.
Clinical characteristics of LFS
Germline TP53 pathogenic variants were identified in 17% (n = 91) of 525 samples submitted to City of Hope laboratories for clinical TP53 testing.[
Subsequently, a large clinical series of patients from France who were tested primarily based on the 2009 version of the Chompret criteria [
Similarly, results of 286 TP53 pathogenic variant–positive individuals in the National Cancer Institute's LFS Study indicated a cumulative cancer incidence of almost 100% by age 70 years for both males and females.[
| | Cumulative Cancer Risk by Age 70 Years | |
|---|---|---|
| a Adapted from Mai et al.[ |
||
| b Other cancers, such as adrenocortical carcinoma, leukemia, and lung bronchoalveolar cancer, have been considered part of the LFS cancer spectrum.[ |
||
| Cancer Type | Females (%) | Males (%) |
| Breast cancer | 54 | – |
| Soft tissue sarcoma | 15 | 22 |
| Brain cancer | 6 | 19 |
| Osteosarcoma | 5 | 11 |
With the increasing use of multigene (panel) tests, it is important to recognize that pathogenic variants in TP53 are unexpectedly being identified in individuals without a family history characteristic of LFS.[
One cohort study evaluated 116 individuals with a germline TP53 pathogenic variant yearly at the National Institutes of Health Clinical Center using multimodality screening with and without gadolinium. Baseline screening identified a cancer in eight patients (6.9%) with a false-positive rate of 34.5% for MRI (n = 40).[
PTENHamartoma Tumor Syndromes (Including Cowden Syndrome)
Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome (BRRS) are part of a spectrum of conditions known collectively as PTEN hamartoma tumor syndromes (PHTS). Approximately 85% of patients diagnosed with Cowden syndrome, and approximately 60% of patients with BRRS have an identifiable PTEN pathogenic variant.[
PTEN functions as a dual-specificity phosphatase that removes phosphate groups from tyrosine, serine, and threonine. PTEN pathogenic variants are diverse and can present as nonsense, missense, frameshift, or splice-site variants. Approximately 40% of variants are found in exon 5, which encodes the phosphatase core motif; several recurrent pathogenic variants have been observed at this location.[
Operational criteria for the diagnosis of Cowden syndrome have been published and subsequently updated.[
Over a 10-year period, the International Cowden Consortium (ICC) prospectively recruited a consecutive series of adult and pediatric patients meeting relaxed ICC criteria for PTEN testing in the United States, Europe, and Asia.[
Although PTEN pathogenic variants, which are estimated to occur in 1 in 200,000 individuals,[
Hereditary Diffuse Gastric Cancer (HDGC)
For more information about HDGC, see the following:
- For general information about HDGC, see
Hereditary Diffuse Gastric Cancer . - For information about HDGC-associated lobular breast cancer, see the
Breast Cancer Risk section in Hereditary Diffuse Gastric Cancer. - For information about management of breast cancer risk in HDGC, see the
Breast Cancer Risk Management section in Hereditary Diffuse Gastric Cancer.
Peutz-Jeghers Syndrome (PJS)
PJS is an early-onset autosomal dominant disorder characterized by melanocytic macules on the lips, the perioral region, and buccal region; and multiple GI polyps, both hamartomatous and adenomatous.[
Females with PJS are also predisposed to the development of cervical adenoma malignum, a rare and very aggressive adenocarcinoma of the cervix.[
Although the risk of malignancy appears to be exceedingly high in individuals with PJS based on the published literature, the possibility that selection and referral biases have resulted in overestimates of these risks should be considered.
| Site | Age (y) | Cumulative Risk (%)b | Reference(s) |
|---|---|---|---|
| GI = gastrointestinal. | |||
| a Reprinted with permission from Macmillan Publishers Ltd: Gastroenterology[ |
|||
| b All cumulative risks were increased compared with the general population (P< .05), with the exception of cervix and testes. | |||
| c GI cancers include colorectal, small intestinal, gastric, esophageal, and pancreatic. | |||
| d Westerman et al.: GI cancer does not include pancreatic cancer.[ |
|||
| e Did not include adenoma malignum of the cervix or Sertoli cell tumors of the testes. | |||
| Any cancer | 60–70 | 37–93 | [ |
| Any GI cancerc,d | 60–70 | 38–66 | [ |
| Gynecological cancer | 60–70 | 13–18 | [ |
| Per origin | |||
| Stomach | 65 | 29 | [ |
| Small bowel | 65 | 13 | [ |
| Colorectum | 65 | 39 | [ |
| Pancreas | 65–70 | 11–36 | [ |
| Lung | 65–70 | 7–17 | [ |
| Breast | 60–70 | 32–54 | [ |
| Uterus | 65 | 9 | [ |
| Ovary | 65 | 21 | [ |
| Cervixe | 65 | 10 | [ |
| Testese | 65 | 9 | [ |
PJS is caused by pathogenic variants in the STK11 (also called LKB1) tumor suppressor gene located on chromosome 19p13.[
Germline variants of the STK11 gene represent a spectrum of nonsense, frameshift, and missense variants, and splice-site variants and large deletions.[
Approximately 85% of variants are localized to regions of the kinase domain of the expressed protein. No strong genotype-phenotype correlations have been identified.[
STK11 has been unequivocally demonstrated to cause PJS. Although earlier estimates using direct DNA sequencing showed a 50% pathogenic variant detection rate in STK11, studies adding techniques to detect large deletions have found pathogenic variants in up to 94% of individuals meeting clinical criteria for PJS.[
Clinical management
NCCN and the U.S. Multi-Society Task Force (USMSTF) on Colorectal Cancer recommend upper endoscopy and high-quality colonoscopy with polypectomy beginning between the ages of 8 to 10 years.[
Management of small bowel hamartomas is important because patients with PJS have risks of bleeding, intussusception, and malignancy. In PJS, cumulative lifetime risk of small bowel cancer is approximately 13%. NCCN guidelines recommend computed tomography enterography (CTE), magnetic resonance enterography (MRE), or video capsule endoscopy (VCE) beginning between the ages of 8 to 10 years for small bowel surveillance in PJS.[
PALB2
Pathogenic variants in the PALB2 gene are associated with increased risks of breast, pancreatic, and ovarian cancers. For more information about PALB2 cancer risks and management options, see
De Novo Pathogenic Variant Rate
Until the 1990s, the diagnosis of genetically inherited breast and ovarian cancer syndromes was based on clinical manifestations and family history. Now that some of the genes involved in these syndromes have been identified, a few studies have attempted to estimate the spontaneous pathogenic variant rate (de novo pathogenic variant rate) in these populations. Interestingly, PJS, PTEN hamartoma syndromes, and LFS are all thought to have high rates of spontaneous pathogenic variants, in the 10% to 30% range,[
References:
- Vasen HF: Clinical description of the Lynch syndrome [hereditary nonpolyposis colorectal cancer (HNPCC)]. Fam Cancer 4 (3): 219-25, 2005.
- Jascur T, Boland CR: Structure and function of the components of the human DNA mismatch repair system. Int J Cancer 119 (9): 2030-5, 2006.
- Papadopoulos N, Nicolaides NC, Wei YF, et al.: Mutation of a mutL homolog in hereditary colon cancer. Science 263 (5153): 1625-9, 1994.
- Peltomäki P, Vasen HF: Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology 113 (4): 1146-58, 1997.
- Akiyama Y, Sato H, Yamada T, et al.: Germ-line mutation of the hMSH6/GTBP gene in an atypical hereditary nonpolyposis colorectal cancer kindred. Cancer Res 57 (18): 3920-3, 1997.
- Miyaki M, Konishi M, Tanaka K, et al.: Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet 17 (3): 271-2, 1997.
- Huang J, Kuismanen SA, Liu T, et al.: MSH6 and MSH3 are rarely involved in genetic predisposition to nonpolypotic colon cancer. Cancer Res 61 (4): 1619-23, 2001.
- Nicolaides NC, Papadopoulos N, Liu B, et al.: Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 371 (6492): 75-80, 1994.
- Hendriks YM, Jagmohan-Changur S, van der Klift HM, et al.: Heterozygous mutations in PMS2 cause hereditary nonpolyposis colorectal carcinoma (Lynch syndrome). Gastroenterology 130 (2): 312-22, 2006.
- Worthley DL, Walsh MD, Barker M, et al.: Familial mutations in PMS2 can cause autosomal dominant hereditary nonpolyposis colorectal cancer. Gastroenterology 128 (5): 1431-6, 2005.
- Vasen HF, Mecklin JP, Khan PM, et al.: The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 34 (5): 424-5, 1991.
- Vasen HF, Watson P, Mecklin JP, et al.: New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 116 (6): 1453-6, 1999.
- Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al.: A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst 89 (23): 1758-62, 1997.
- Umar A, Boland CR, Terdiman JP, et al.: Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96 (4): 261-8, 2004.
- Watson P, Lynch HT: Cancer risk in mismatch repair gene mutation carriers. Fam Cancer 1 (1): 57-60, 2001.
- Vasen HF, Wijnen JT, Menko FH, et al.: Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology 110 (4): 1020-7, 1996.
- Aarnio M, Mecklin JP, Aaltonen LA, et al.: Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer 64 (6): 430-3, 1995.
- Watson P, Lynch HT: Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer 71 (3): 677-85, 1993.
- Brown GJ, St John DJ, Macrae FA, et al.: Cancer risk in young women at risk of hereditary nonpolyposis colorectal cancer: implications for gynecologic surveillance. Gynecol Oncol 80 (3): 346-9, 2001.
- Aarnio M, Sankila R, Pukkala E, et al.: Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 81 (2): 214-8, 1999.
- Ten Broeke SW, van der Klift HM, Tops CMJ, et al.: Cancer Risks for PMS2-Associated Lynch Syndrome. J Clin Oncol 36 (29): 2961-2968, 2018.
- Møller P, Seppälä TT, Bernstein I, et al.: Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 67 (7): 1306-1316, 2018.
- Grindedal EM, Renkonen-Sinisalo L, Vasen H, et al.: Survival in women with MMR mutations and ovarian cancer: a multicentre study in Lynch syndrome kindreds. J Med Genet 47 (2): 99-102, 2010.
- Pal T, Permuth-Wey J, Sellers TA: A review of the clinical relevance of mismatch-repair deficiency in ovarian cancer. Cancer 113 (4): 733-42, 2008.
- Jensen UB, Sunde L, Timshel S, et al.: Mismatch repair defective breast cancer in the hereditary nonpolyposis colorectal cancer syndrome. Breast Cancer Res Treat 120 (3): 777-82, 2010.
- Shanley S, Fung C, Milliken J, et al.: Breast cancer immunohistochemistry can be useful in triage of some HNPCC families. Fam Cancer 8 (3): 251-5, 2009.
- Walsh MD, Buchanan DD, Cummings MC, et al.: Lynch syndrome-associated breast cancers: clinicopathologic characteristics of a case series from the colon cancer family registry. Clin Cancer Res 16 (7): 2214-24, 2010.
- Buerki N, Gautier L, Kovac M, et al.: Evidence for breast cancer as an integral part of Lynch syndrome. Genes Chromosomes Cancer 51 (1): 83-91, 2012.
- Win AK, Young JP, Lindor NM, et al.: Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol 30 (9): 958-64, 2012.
- Win AK, Lindor NM, Young JP, et al.: Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst 104 (18): 1363-72, 2012.
- Harkness EF, Barrow E, Newton K, et al.: Lynch syndrome caused by MLH1 mutations is associated with an increased risk of breast cancer: a cohort study. J Med Genet 52 (8): 553-6, 2015.
- Win AK, Lindor NM, Jenkins MA: Risk of breast cancer in Lynch syndrome: a systematic review. Breast Cancer Res 15 (2): R27, 2013.
- Goldberg M, Bell K, Aronson M, et al.: Association between the Lynch syndrome gene MSH2 and breast cancer susceptibility in a Canadian familial cancer registry. J Med Genet 54 (11): 742-746, 2017.
- Roberts ME, Jackson SA, Susswein LR, et al.: MSH6 and PMS2 germ-line pathogenic variants implicated in Lynch syndrome are associated with breast cancer. Genet Med 20 (10): 1167-1174, 2018.
- Espenschied CR, LaDuca H, Li S, et al.: Multigene Panel Testing Provides a New Perspective on Lynch Syndrome. J Clin Oncol 35 (22): 2568-2575, 2017.
- Lu HM, Li S, Black MH, et al.: Association of Breast and Ovarian Cancers With Predisposition Genes Identified by Large-Scale Sequencing. JAMA Oncol 5 (1): 51-57, 2019.
- Latham A, Srinivasan P, Kemel Y, et al.: Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. J Clin Oncol 37 (4): 286-295, 2019.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 1.2021. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2020.
Available online with free registration. Last accessed May 24, 2024. - Harris CC, Hollstein M: Clinical implications of the p53 tumor-suppressor gene. N Engl J Med 329 (18): 1318-27, 1993.
- Malkin D: The Li-Fraumeni syndrome. Cancer: Principles and Practice of Oncology Updates 7(7): 1-14, 1993.
- Gonzalez KD, Noltner KA, Buzin CH, et al.: Beyond Li Fraumeni Syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 27 (8): 1250-6, 2009.
- Chompret A, Abel A, Stoppa-Lyonnet D, et al.: Sensitivity and predictive value of criteria for p53 germline mutation screening. J Med Genet 38 (1): 43-7, 2001.
- Tinat J, Bougeard G, Baert-Desurmont S, et al.: 2009 version of the Chompret criteria for Li Fraumeni syndrome. J Clin Oncol 27 (26): e108-9; author reply e110, 2009.
- Bottomley RH, Condit PT: Cancer families. Cancer Bull 20: 22-24, 1968.
- Bougeard G, Renaux-Petel M, Flaman JM, et al.: Revisiting Li-Fraumeni Syndrome From TP53 Mutation Carriers. J Clin Oncol 33 (21): 2345-52, 2015.
- Sidransky D, Tokino T, Helzlsouer K, et al.: Inherited p53 gene mutations in breast cancer. Cancer Res 52 (10): 2984-6, 1992.
- Wilson JR, Bateman AC, Hanson H, et al.: A novel HER2-positive breast cancer phenotype arising from germline TP53 mutations. J Med Genet 47 (11): 771-4, 2010.
- Melhem-Bertrandt A, Bojadzieva J, Ready KJ, et al.: Early onset HER2-positive breast cancer is associated with germline TP53 mutations. Cancer 118 (4): 908-13, 2012.
- Masciari S, Dillon DA, Rath M, et al.: Breast cancer phenotype in women with TP53 germline mutations: a Li-Fraumeni syndrome consortium effort. Breast Cancer Res Treat 133 (3): 1125-30, 2012.
- Pearson AD, Craft AW, Ratcliffe JM, et al.: Two families with the Li-Fraumeni cancer family syndrome. J Med Genet 19 (5): 362-5, 1982.
- Li FP, Fraumeni JF, Mulvihill JJ, et al.: A cancer family syndrome in twenty-four kindreds. Cancer Res 48 (18): 5358-62, 1988.
- Bougeard G, Sesboüé R, Baert-Desurmont S, et al.: Molecular basis of the Li-Fraumeni syndrome: an update from the French LFS families. J Med Genet 45 (8): 535-8, 2008.
- Mai PL, Best AF, Peters JA, et al.: Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 122 (23): 3673-3681, 2016.
- Kamihara J, Rana HQ, Garber JE: Germline TP53 mutations and the changing landscape of Li-Fraumeni syndrome. Hum Mutat 35 (6): 654-62, 2014.
- Mai PL, Khincha PP, Loud JT, et al.: Prevalence of Cancer at Baseline Screening in the National Cancer Institute Li-Fraumeni Syndrome Cohort. JAMA Oncol 3 (12): 1640-1645, 2017.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023.
Available online with free registration. Last accessed September 18, 2024. - Villani A, Tabori U, Schiffman J, et al.: Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: a prospective observational study. Lancet Oncol 12 (6): 559-67, 2011.
- Masciari S, Van den Abbeele AD, Diller LR, et al.: F18-fluorodeoxyglucose-positron emission tomography/computed tomography screening in Li-Fraumeni syndrome. JAMA 299 (11): 1315-9, 2008.
- Zhou XP, Waite KA, Pilarski R, et al.: Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am J Hum Genet 73 (2): 404-11, 2003.
- Mester J, Eng C: When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 163C (2): 114-21, 2013.
- Eng C: PTEN: one gene, many syndromes. Hum Mutat 22 (3): 183-98, 2003.
- Marsh DJ, Kum JB, Lunetta KL, et al.: PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet 8 (8): 1461-72, 1999.
- Pilarski R, Eng C: Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. J Med Genet 41 (5): 323-6, 2004.
- Eng C: PTEN Hamartoma Tumor Syndrome (PHTS). In: Adam MP, Feldman J, Mirzaa GM, et al., eds.: GeneReviews. University of Washington, Seattle, 1993-2024, pp.
Available online . Last accessed November 13, 2024. - Pilarski R, Burt R, Kohlman W, et al.: Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst 105 (21): 1607-16, 2013.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2022. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2022.
Available online with free registration. Last accessed May 24, 2024. - Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015.
- Ngeow J, Liu C, Zhou K, et al.: Detecting Germline PTEN Mutations Among At-Risk Patients With Cancer: An Age- and Sex-Specific Cost-Effectiveness Analysis. J Clin Oncol 33 (23): 2537-44, 2015.
- Tan MH, Mester JL, Ngeow J, et al.: Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 18 (2): 400-7, 2012.
- Bubien V, Bonnet F, Brouste V, et al.: High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet 50 (4): 255-63, 2013.
- Myers MP, Tonks NK: PTEN: sometimes taking it off can be better than putting it on. Am J Hum Genet 61 (6): 1234-8, 1997.
- Hobert JA, Eng C: PTEN hamartoma tumor syndrome: an overview. Genet Med 11 (10): 687-94, 2009.
- Nieuwenhuis MH, Kets CM, Murphy-Ryan M, et al.: Cancer risk and genotype-phenotype correlations in PTEN hamartoma tumor syndrome. Fam Cancer 13 (1): 57-63, 2014.
- Ngeow J, Stanuch K, Mester JL, et al.: Second malignant neoplasms in patients with Cowden syndrome with underlying germline PTEN mutations. J Clin Oncol 32 (17): 1818-24, 2014.
- Olopade OI, Weber BL: Breast cancer genetics: toward molecular characterization of individuals at increased risk for breast cancer: part I. Cancer: Principles and Practice of Oncology Updates 12 (10): 1-12, 1998.
- Pilarski R, Stephens JA, Noss R, et al.: Predicting PTEN mutations: an evaluation of Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome clinical features. J Med Genet 48 (8): 505-12, 2011.
- Nelen MR, Padberg GW, Peeters EA, et al.: Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet 13 (1): 114-6, 1996.
- Lachlan KL, Lucassen AM, Bunyan D, et al.: Cowden syndrome and Bannayan Riley Ruvalcaba syndrome represent one condition with variable expression and age-related penetrance: results of a clinical study of PTEN mutation carriers. J Med Genet 44 (9): 579-85, 2007.
- Peutz JL: Very remarkable case of familial polyposis of mucous membrane of intestinal tract and nasopharynx accompanied by peculiar pigmentations of skin and mucous membrane. Ned Tijdschr Geneeskd 10: 134-146, 1921.
- Jeghers H, McKusick VA, Katz KH: Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J Med 241 (26): 1031-6, 1949.
- Spigelman AD, Murday V, Phillips RK: Cancer and the Peutz-Jeghers syndrome. Gut 30 (11): 1588-90, 1989.
- Aretz S, Stienen D, Uhlhaas S, et al.: High proportion of large genomic STK11 deletions in Peutz-Jeghers syndrome. Hum Mutat 26 (6): 513-9, 2005.
- Hemminki A, Markie D, Tomlinson I, et al.: A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391 (6663): 184-7, 1998.
- Jenne DE, Reimann H, Nezu J, et al.: Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 18 (1): 38-43, 1998.
- Boudeau J, Kieloch A, Alessi DR, et al.: Functional analysis of LKB1/STK11 mutants and two aberrant isoforms found in Peutz-Jeghers Syndrome patients. Hum Mutat 21 (2): 172, 2003.
- Lim W, Hearle N, Shah B, et al.: Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br J Cancer 89 (2): 308-13, 2003.
- Giardiello FM, Brensinger JD, Tersmette AC, et al.: Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 119 (6): 1447-53, 2000.
- Hearle N, Schumacher V, Menko FH, et al.: Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 12 (10): 3209-15, 2006.
- Lim W, Olschwang S, Keller JJ, et al.: Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology 126 (7): 1788-94, 2004.
- van Lier MG, Wagner A, Mathus-Vliegen EM, et al.: High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol 105 (6): 1258-64; author reply 1265, 2010.
- Srivatsa PJ, Keeney GL, Podratz KC: Disseminated cervical adenoma malignum and bilateral ovarian sex cord tumors with annular tubules associated with Peutz-Jeghers syndrome. Gynecol Oncol 53 (2): 256-64, 1994.
- Scully RE: Sex cord tumor with annular tubules a distinctive ovarian tumor of the Peutz-Jeghers syndrome. Cancer 25 (5): 1107-21, 1970.
- Westerman AM, Entius MM, de Baar E, et al.: Peutz-Jeghers syndrome: 78-year follow-up of the original family. Lancet 353 (9160): 1211-5, 1999.
- Mehenni H, Resta N, Park JG, et al.: Cancer risks in LKB1 germline mutation carriers. Gut 55 (7): 984-90, 2006.
- Gruber SB, Entius MM, Petersen GM, et al.: Pathogenesis of adenocarcinoma in Peutz-Jeghers syndrome. Cancer Res 58 (23): 5267-70, 1998.
- Wang ZJ, Ellis I, Zauber P, et al.: Allelic imbalance at the LKB1 (STK11) locus in tumours from patients with Peutz-Jeghers' syndrome provides evidence for a hamartoma-(adenoma)-carcinoma sequence. J Pathol 188 (1): 9-13, 1999.
- Miyoshi H, Nakau M, Ishikawa TO, et al.: Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res 62 (8): 2261-6, 2002.
- Nakau M, Miyoshi H, Seldin MF, et al.: Hepatocellular carcinoma caused by loss of heterozygosity in Lkb1 gene knockout mice. Cancer Res 62 (16): 4549-53, 2002.
- Takeda H, Miyoshi H, Kojima Y, et al.: Accelerated onsets of gastric hamartomas and hepatic adenomas/carcinomas in Lkb1+/-p53-/- compound mutant mice. Oncogene 25 (12): 1816-20, 2006.
- Amos CI, Keitheri-Cheteri MB, Sabripour M, et al.: Genotype-phenotype correlations in Peutz-Jeghers syndrome. J Med Genet 41 (5): 327-33, 2004.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal. Version 2.2023 . Plymouth Meeting, PA: National Comprehensive Cancer Network, 2023.
Available with free registration. Last accessed March 13, 2024. - Boland CR, Idos GE, Durno C, et al.: Diagnosis and Management of Cancer Risk in the Gastrointestinal Hamartomatous Polyposis Syndromes: Recommendations From the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 162 (7): 2063-2085, 2022.
- Urquhart P, Grimpen F, Lim GJ, et al.: Capsule endoscopy versus magnetic resonance enterography for the detection of small bowel polyps in Peutz-Jeghers syndrome. Fam Cancer 13 (2): 249-55, 2014.
- Westerman AM, Entius MM, Boor PP, et al.: Novel mutations in the LKB1/STK11 gene in Dutch Peutz-Jeghers families. Hum Mutat 13 (6): 476-81, 1999.
- Schreibman IR, Baker M, Amos C, et al.: The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol 100 (2): 476-90, 2005.
- Gonzalez KD, Buzin CH, Noltner KA, et al.: High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 46 (10): 689-93, 2009.
- Bendig I, Mohr N, Kramer F, et al.: Identification of novel TP53 mutations in familial and sporadic cancer cases of German and Swiss origin. Cancer Genet Cytogenet 154 (1): 22-6, 2004.
- De Leeneer K, Coene I, Crombez B, et al.: Prevalence of BRCA1/2 mutations in sporadic breast/ovarian cancer patients and identification of a novel de novo BRCA1 mutation in a patient diagnosed with late onset breast and ovarian cancer: implications for genetic testing. Breast Cancer Res Treat 132 (1): 87-95, 2012.
- Diez O, Gutiérrez-Enríquez S, Mediano C, et al.: A novel de novo BRCA2 mutation of paternal origin identified in a Spanish woman with early onset bilateral breast cancer. Breast Cancer Res Treat 121 (1): 221-5, 2010.
- Garcia-Casado Z, Romero I, Fernandez-Serra A, et al.: A de novo complete BRCA1 gene deletion identified in a Spanish woman with early bilateral breast cancer. BMC Med Genet 12: 134, 2011.
- Hansen TV, Bisgaard ML, Jønson L, et al.: Novel de novo BRCA2 mutation in a patient with a family history of breast cancer. BMC Med Genet 9: 58, 2008.
- Kwong A, Ng EK, Tang EY, et al.: A novel de novo BRCA1 mutation in a Chinese woman with early onset breast cancer. Fam Cancer 10 (2): 233-7, 2011.
- Marshall M, Solomon S, Lawrence Wickerham D: Case report: de novo BRCA2 gene mutation in a 35-year-old woman with breast cancer. Clin Genet 76 (5): 427-30, 2009.
- Robson M, Scheuer L, Nafa K, et al.: Unique de novo mutation of BRCA2 in a woman with early onset breast cancer. J Med Genet 39 (2): 126-8, 2002.
- Tesoriero A, Andersen C, Southey M, et al.: De novo BRCA1 mutation in a patient with breast cancer and an inherited BRCA2 mutation. Am J Hum Genet 65 (2): 567-9, 1999.
- van der Luijt RB, van Zon PH, Jansen RP, et al.: De novo recurrent germline mutation of the BRCA2 gene in a patient with early onset breast cancer. J Med Genet 38 (2): 102-5, 2001.
- Morak M, Laner A, Scholz M, et al.: Report on de-novo mutation in the MSH2 gene as a rare event in hereditary nonpolyposis colorectal cancer. Eur J Gastroenterol Hepatol 20 (11): 1101-5, 2008.
- Plasilova M, Zhang J, Okhowat R, et al.: A de novo MLH1 germ line mutation in a 31-year-old colorectal cancer patient. Genes Chromosomes Cancer 45 (12): 1106-10, 2006.
- Win AK, Jenkins MA, Buchanan DD, et al.: Determining the frequency of de novo germline mutations in DNA mismatch repair genes. J Med Genet 48 (8): 530-4, 2011.
- Anderson KG: How well does paternity confidence match actual paternity? Evidence from worldwide nonpaternity rates. Curr Anthropol 47 (3): 513-20, 2006.
Also available online . Last accessed May 24, 2024. - Sasse G, Müller H, Chakraborty R, et al.: Estimating the frequency of nonpaternity in Switzerland. Hum Hered 44 (6): 337-43, 1994 Nov-Dec.
- Voracek M, Haubner T, Fisher ML: Recent decline in nonpaternity rates: a cross-temporal meta-analysis. Psychol Rep 103 (3): 799-811, 2008.
Moderate-Penetrance Genes Associated With Breast and / or Gynecologic Cancers
Background
Pathogenic variants in BRCA1, BRCA2, PALB2, and the genes involved in other rare syndromes discussed in the
Breast and Gynecologic Cancer Susceptibility Genes Identified Through Candidate Gene Approaches
There is a very large literature of genetic epidemiology studies describing associations between various loci and breast cancer risk. Many of these studies suffer from significant design limitations. Perhaps as a consequence, most reported associations do not replicate in follow-up studies. This section is not a comprehensive review of all reported associations. This section describes associations that are believed by the editors to be clinically valid, in that they have been described in several studies or are supported by robust meta-analyses. The clinical utility of these observations remains unclear, however, as the risks associated with these variations usually fall below a threshold that would justify a clinical response.
Fanconi anemia genes
Fanconi anemia (FA) is a rare, inherited condition characterized by bone marrow failure, increased risk of malignancy, and physical abnormalities. To date, 16 FA-related genes, including
| a Refer to the |
| b Refer to the |
| c Moderate risk is defined as a statistically significant, twofold or lower increased risk estimate. |
| High-Risk Genes |
| –BRCA1(FANCS)a |
| –BRCA2(FANCD1)a |
| –PALB2(FANCN)b |
| Moderate-Risk Genes c |
| –BRIP1(FANCJ/BACH1) |
| –FANCD2 |
| –RAD51C(FANCO) |
| Genes With Uncertain or No Significantly Increased Risk |
| –FANCA |
| –FANCB |
| –FANCC |
| –FANCE |
| –FANCF |
| –FANCG(XRCC9) |
| –FANCI(KIAA1794) |
| –FANCL |
| –SLX4(FANCP) |
| –ERCC4(FANCQ/XPF) |
Progressive bone marrow failure typically occurs in the first decade, with patients often presenting with thrombocytopenia or leucopenia. The incidence of bone marrow failure is 90% by age 40 to 50 years. The incidence is 10% to 30% for hematologic malignancies (primarily acute myeloid leukemia) and 25% to 30% for nonhematologic malignancies (solid tumors, particularly of the head and neck, skin, gastrointestinal [GI] tract, and genital tract). Physical abnormalities, including short stature, abnormal skin pigmentation, radial ray defects (including malformation of the thumbs), abnormalities of the urinary tract, eyes, ears, heart, GI system, and central nervous system, hypogonadism, and developmental delay are present in 60% to 75% of affected individuals.[
Variants in some of the FA genes, most notably BRCA1 and BRCA2, but also PALB2, RAD51C, and BRIP1, among others, may predispose to breast cancer in heterozygotes. Given the widespread availability of multigene (panel) tests, genetic testing of many of the FA genes is frequently performed despite uncertain cancer risks and the lack of available evidence-based medical management recommendations for many of these genes.
FA gene pathogenic variant carrier status can have implications for reproductive decision making because pathogenic variants in these genes can lead to serious childhood onset of disease if both parents are carriers of pathogenic variants in the same gene. Partner testing may be considered.
BRIP1
BRIP1 (also known as BACH1) encodes a helicase that interacts with the BRCA1 C-terminal domain. This gene also has a role in BRCA1-dependent DNA repair and cell cycle checkpoint function. Biallelic pathogenic variants in BRIP1 are a cause of FA,[
Monoallelic pathogenic variants in BRIP1 have emerged as having a significant association with increased ovarian cancer risk. Nine-tenths to two and half percent of women with ovarian cancer carry a pathogenic variant in BRIP1.[
With respect to breast cancer risk, several studies consistently report ORs less than 2.0. A meta-analysis of 148 studies found an OR for breast cancer of 1.62 in individuals with BRIP1 pathogenic variants (95% confidence interval [CI], 1.20–2.20).[
CHEK2
CHEK2 is a gene involved in the DNA damage repair response pathway. Based on numerous studies, a polymorphism, 1100delC, appears to be a rare, moderate-penetrance cancer susceptibility allele.[
Two studies have suggested that the risk associated with a CHEK2 1100delC pathogenic variant was stronger in the families of probands ascertained because of bilateral breast cancer.[
A large Dutch study of 86,975 individuals reported an increased risk of cancers other than breast and colon for carriers of the CHEK2 1100delC pathogenic variant,[
(Refer to the
ATM
Ataxia telangiectasia (AT) is an autosomal recessive disorder characterized by neurologic deterioration, telangiectasias, immunodeficiency states, and hypersensitivity to ionizing radiation. It is estimated that 1% of the general population may be heterozygote carriers of ATM variants.[
Initial, large epidemiological studies demonstrated a statistically increased relative risk (RR) of approximately 2.0 for breast cancer among female ATM heterozygotes.[
Age-specific cumulative breast cancer risks modeled through a meta-analysis were reported to be 6.02% by age 50 years and 32.83% by age 80 years.[
While multiple studies have reported that most ATM pathogenic variants impart moderate risks for breast cancer, the c.7271T>G missense variant has been shown to predispose individuals to higher breast cancer risks.[
Some studies reported an association between ATM and ovarian cancer, with ovarian cancer lifetime risk approaching ~3%.[
Pancreatic cancer has also been associated with ATM pathogenic variants, with an OR of 4.21 (95% CI, 3.24–5.47) reported through a commercial lab–based study.[
The association between ATM pathogenic variants and prostate cancer risk have been inconclusive, with a commercial lab–based study reporting an OR of 2.58 (95% CI, 1.93–3.44).[
RAD51
RAD51 and the family of RAD51-related genes, also known as RAD51 paralogs, are thought to encode proteins that are involved in DNA damage repair through homologous recombination and interaction with numerous other DNA repair proteins, including BRCA1 and BRCA2. The RAD51 protein plays a central role in single-strand annealing in the DNA damage response. RAD51 recruitment to break sites and recombinational DNA repair depend on the RAD51 paralogs, although their precise cellular functions are poorly characterized.[
One of five RAD51-related genes, RAD51C has been reported to be linked to both FA-like disorders and familial breast and ovarian cancers. The literature, however, has produced contradictory findings. In a study of 480 German families characterized by breast and ovarian cancers who were negative for BRCA1 and BRCA2 pathogenic variants, six monoallelic variants in RAD51C were found (frequency of 1.3%).[
In addition to carriers of RAD51C pathogenic variants, there are other RAD51 paralogs, including RAD51B, RAD51D, RAD51L1, XRCC2, and XRCC3, that may be associated with breast and/or ovarian cancer risk,[
In addition to germline variants, different polymorphisms of RAD51 have been hypothesized to have reduced capacity to repair DNA defects, resulting in increased susceptibility to familial breast cancer. The Consortium of Investigators of Modifiers of BRCA1/BRCA2 (CIMBA) pooled data from 8,512 carriers of BRCA1 and BRCA2 pathogenic variants and found evidence of an increased risk of breast cancer among women who were BRCA2 carriers and who were homozygous for CC at the RAD51 135G→C SNV (hazard ratio, 1.17; 95% CI, 0.91–1.51).[
Several meta-analyses have investigated the association between the RAD51 135G→C polymorphism and breast cancer risk. There is significant overlap in the studies reported in these meta-analyses, significant variability in the characteristics of the populations included, and significant methodologic limitations to their findings.[
In summary, among this conflicting data is substantial evidence for a modest association between germline variants in RAD51C and breast cancer and ovarian cancer. There is also evidence of an association between polymorphisms in RAD51 135G→C among women with homozygous CC genotypes and breast cancer, particularly among BRCA2 carriers. These associations are plausible given the known role of RAD51 in the maintenance of genomic stability.
References:
- Easton DF: How many more breast cancer predisposition genes are there? Breast Cancer Res 1 (1): 14-7, 1999.
- Smith P, McGuffog L, Easton DF, et al.: A genome wide linkage search for breast cancer susceptibility genes. Genes Chromosomes Cancer 45 (7): 646-55, 2006.
- Cybulski C, Wokołorczyk D, Jakubowska A, et al.: Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol 29 (28): 3747-52, 2011.
- Thompson D, Duedal S, Kirner J, et al.: Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst 97 (11): 813-22, 2005.
- Pelttari LM, Heikkinen T, Thompson D, et al.: RAD51C is a susceptibility gene for ovarian cancer. Hum Mol Genet 20 (16): 3278-88, 2011.
- Loveday C, Turnbull C, Ramsay E, et al.: Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet 43 (9): 879-82, 2011.
- Mehta PA, Tolar J: Fanconi Anemia. In: Adam MP, Feldman J, Mirzaa GM, et al., eds.: GeneReviews. University of Washington, Seattle, 1993-2024, pp.
Available online. Last accessed November 8, 2023. - Levitus M, Waisfisz Q, Godthelp BC, et al.: The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet 37 (9): 934-5, 2005.
- Levran O, Attwooll C, Henry RT, et al.: The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat Genet 37 (9): 931-3, 2005.
- Litman R, Peng M, Jin Z, et al.: BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell 8 (3): 255-65, 2005.
- Moyer CL, Ivanovich J, Gillespie JL, et al.: Rare BRIP1 Missense Alleles Confer Risk for Ovarian and Breast Cancer. Cancer Res 80 (4): 857-867, 2020.
- Suszynska M, Ratajska M, Kozlowski P: BRIP1, RAD51C, and RAD51D mutations are associated with high susceptibility to ovarian cancer: mutation prevalence and precise risk estimates based on a pooled analysis of ~30,000 cases. J Ovarian Res 13 (1): 50, 2020.
- Lhotova K, Stolarova L, Zemankova P, et al.: Multigene Panel Germline Testing of 1333 Czech Patients with Ovarian Cancer. Cancers (Basel) 12 (4): , 2020.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023.
Available online with free registration. Last accessed September 18, 2024. - Alter BP, Best AF: Frequency of heterozygous germline pathogenic variants in genes for Fanconi anemia in patients with non-BRCA1/BRCA2 breast cancer: a meta-analysis. Breast Cancer Res Treat 182 (2): 465-476, 2020.
- Meijers-Heijboer H, van den Ouweland A, Klijn J, et al.: Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31 (1): 55-9, 2002.
- Kuschel B, Auranen A, Gregory CS, et al.: Common polymorphisms in checkpoint kinase 2 are not associated with breast cancer risk. Cancer Epidemiol Biomarkers Prev 12 (8): 809-12, 2003.
- Sodha N, Bullock S, Taylor R, et al.: CHEK2 variants in susceptibility to breast cancer and evidence of retention of the wild type allele in tumours. Br J Cancer 87 (12): 1445-8, 2002.
- Ingvarsson S, Sigbjornsdottir BI, Huiping C, et al.: Mutation analysis of the CHK2 gene in breast carcinoma and other cancers. Breast Cancer Res 4 (3): R4, 2002.
- Vahteristo P, Bartkova J, Eerola H, et al.: A CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet 71 (2): 432-8, 2002.
- Meijers-Heijboer H, Wijnen J, Vasen H, et al.: The CHEK2 1100delC mutation identifies families with a hereditary breast and colorectal cancer phenotype. Am J Hum Genet 72 (5): 1308-14, 2003.
- Schmidt MK, Tollenaar RA, de Kemp SR, et al.: Breast cancer survival and tumor characteristics in premenopausal women carrying the CHEK2*1100delC germline mutation. J Clin Oncol 25 (1): 64-9, 2007.
- Weischer M, Bojesen SE, Tybjaerg-Hansen A, et al.: Increased risk of breast cancer associated with CHEK2*1100delC. J Clin Oncol 25 (1): 57-63, 2007.
- Iniesta MD, Gorin MA, Chien LC, et al.: Absence of CHEK2*1100delC mutation in families with hereditary breast cancer in North America. Cancer Genet Cytogenet 202 (2): 136-40, 2010.
- Offit K, Pierce H, Kirchhoff T, et al.: Frequency of CHEK2*1100delC in New York breast cancer cases and controls. BMC Med Genet 4 (1): 1, 2003.
- Oldenburg RA, Kroeze-Jansema K, Kraan J, et al.: The CHEK2*1100delC variant acts as a breast cancer risk modifier in non-BRCA1/BRCA2 multiple-case families. Cancer Res 63 (23): 8153-7, 2003.
- Neuhausen S, Dunning A, Steele L, et al.: Role of CHEK2*1100delC in unselected series of non-BRCA1/2 male breast cancers. Int J Cancer 108 (3): 477-8, 2004.
- Ohayon T, Gal I, Baruch RG, et al.: CHEK2*1100delC and male breast cancer risk in Israel. Int J Cancer 108 (3): 479-80, 2004.
- CHEK2 Breast Cancer Case-Control Consortium: CHEK2*1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet 74 (6): 1175-82, 2004.
- Yang Y, Zhang F, Wang Y, et al.: CHEK2 1100delC variant and breast cancer risk in Caucasians: a meta-analysis based on 25 studies with 29,154 cases and 37,064 controls. Asian Pac J Cancer Prev 13 (7): 3501-5, 2012.
- Hallamies S, Pelttari LM, Poikonen-Saksela P, et al.: CHEK2 c.1100delC mutation is associated with an increased risk for male breast cancer in Finnish patient population. BMC Cancer 17 (1): 620, 2017.
- Johnson N, Fletcher O, Naceur-Lombardelli C, et al.: Interaction between CHEK2*1100delC and other low-penetrance breast-cancer susceptibility genes: a familial study. Lancet 366 (9496): 1554-7, 2005 Oct 29-Nov 4.
- Fletcher O, Johnson N, Dos Santos Silva I, et al.: Family history, genetic testing, and clinical risk prediction: pooled analysis of CHEK2 1100delC in 1,828 bilateral breast cancers and 7,030 controls. Cancer Epidemiol Biomarkers Prev 18 (1): 230-4, 2009.
- Weischer M, Bojesen SE, Ellervik C, et al.: CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol 26 (4): 542-8, 2008.
- Adank MA, Jonker MA, Kluijt I, et al.: CHEK2*1100delC homozygosity is associated with a high breast cancer risk in women. J Med Genet 48 (12): 860-3, 2011.
- Gronwald J, Cybulski C, Piesiak W, et al.: Cancer risks in first-degree relatives of CHEK2 mutation carriers: effects of mutation type and cancer site in proband. Br J Cancer 100 (9): 1508-12, 2009.
- Wasielewski M, den Bakker MA, van den Ouweland A, et al.: CHEK2 1100delC and male breast cancer in the Netherlands. Breast Cancer Res Treat 116 (2): 397-400, 2009.
- Osorio A, Rodríguez-López R, Díez O, et al.: The breast cancer low-penetrance allele 1100delC in the CHEK2 gene is not present in Spanish familial breast cancer population. Int J Cancer 108 (1): 54-6, 2004.
- Syrjäkoski K, Kuukasjärvi T, Auvinen A, et al.: CHEK2 1100delC is not a risk factor for male breast cancer population. Int J Cancer 108 (3): 475-6, 2004.
- Tsou HC, Teng DH, Ping XL, et al.: The role of MMAC1 mutations in early-onset breast cancer: causative in association with Cowden syndrome and excluded in BRCA1-negative cases. Am J Hum Genet 61 (5): 1036-43, 1997.
- Olopade OI, Weber BL: Breast cancer genetics: toward molecular characterization of individuals at increased risk for breast cancer: part I. Cancer: Principles and Practice of Oncology Updates 12 (10): 1-12, 1998.
- Cybulski C, Górski B, Huzarski T, et al.: CHEK2-positive breast cancers in young Polish women. Clin Cancer Res 12 (16): 4832-5, 2006.
- Cybulski C, Huzarski T, Byrski T, et al.: Estrogen receptor status in CHEK2-positive breast cancers: implications for chemoprevention. Clin Genet 75 (1): 72-8, 2009.
- Näslund-Koch C, Nordestgaard BG, Bojesen SE: Increased Risk for Other Cancers in Addition to Breast Cancer for CHEK2*1100delC Heterozygotes Estimated From the Copenhagen General Population Study. J Clin Oncol 34 (11): 1208-16, 2016.
- Savitsky K, Bar-Shira A, Gilad S, et al.: A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268 (5218): 1749-53, 1995.
- Telatar M, Teraoka S, Wang Z, et al.: Ataxia-telangiectasia: identification and detection of founder-effect mutations in the ATM gene in ethnic populations. Am J Hum Genet 62 (1): 86-97, 1998.
- Uhrhammer N, Bay JO, Bignon YJ: Seventh International Workshop on Ataxia-Telangiectasia. Cancer Res 58 (15): 3480-5, 1998.
- Ahmed M, Rahman N: ATM and breast cancer susceptibility. Oncogene 25 (43): 5906-11, 2006.
- Khanna KK, Chenevix-Trench G: ATM and genome maintenance: defining its role in breast cancer susceptibility. J Mammary Gland Biol Neoplasia 9 (3): 247-62, 2004.
- Gilad S, Chessa L, Khosravi R, et al.: Genotype-phenotype relationships in ataxia-telangiectasia and variants. Am J Hum Genet 62 (3): 551-61, 1998.
- Renwick A, Thompson D, Seal S, et al.: ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet 38 (8): 873-5, 2006.
- Dorling L, Carvalho S, Allen J, et al.: Breast Cancer Risk Genes - Association Analysis in More than 113,000 Women. N Engl J Med 384 (5): 428-439, 2021.
- Hu C, Hart SN, Gnanaolivu R, et al.: A Population-Based Study of Genes Previously Implicated in Breast Cancer. N Engl J Med 384 (5): 440-451, 2021.
- Hall MJ, Bernhisel R, Hughes E, et al.: Germline Pathogenic Variants in the Ataxia Telangiectasia Mutated (ATM) Gene are Associated with High and Moderate Risks for Multiple Cancers. Cancer Prev Res (Phila) 14 (4): 433-440, 2021.
- Marabelli M, Cheng SC, Parmigiani G: Penetrance of ATM Gene Mutations in Breast Cancer: A Meta-Analysis of Different Measures of Risk. Genet Epidemiol 40 (5): 425-31, 2016.
- van Os NJ, Roeleveld N, Weemaes CM, et al.: Health risks for ataxia-telangiectasia mutated heterozygotes: a systematic review, meta-analysis and evidence-based guideline. Clin Genet 90 (2): 105-17, 2016.
- Moslemi M, Moradi Y, Dehghanbanadaki H, et al.: The association between ATM variants and risk of breast cancer: a systematic review and meta-analysis. BMC Cancer 21 (1): 27, 2021.
- Bernstein JL, Teraoka S, Southey MC, et al.: Population-based estimates of breast cancer risks associated with ATM gene variants c.7271T>G and c.1066-6T>G (IVS10-6T>G) from the Breast Cancer Family Registry. Hum Mutat 27 (11): 1122-8, 2006.
- Goldgar DE, Healey S, Dowty JG, et al.: Rare variants in the ATM gene and risk of breast cancer. Breast Cancer Res 13 (4): R73, 2011.
- Lu HM, Li S, Black MH, et al.: Association of Breast and Ovarian Cancers With Predisposition Genes Identified by Large-Scale Sequencing. JAMA Oncol 5 (1): 51-57, 2019.
- Pennington KP, Walsh T, Harrell MI, et al.: Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 20 (3): 764-75, 2014.
- Hsu FC, Roberts NJ, Childs E, et al.: Risk of Pancreatic Cancer Among Individuals With Pathogenic Variants in the ATM Gene. JAMA Oncol 7 (11): 1664-1668, 2021.
- Suwaki N, Klare K, Tarsounas M: RAD51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin Cell Dev Biol 22 (8): 898-905, 2011.
- Vaz F, Hanenberg H, Schuster B, et al.: Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet 42 (5): 406-9, 2010.
- Meindl A, Hellebrand H, Wiek C, et al.: Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet 42 (5): 410-4, 2010.
- Clague J, Wilhoite G, Adamson A, et al.: RAD51C germline mutations in breast and ovarian cancer cases from high-risk families. PLoS One 6 (9): e25632, 2011.
- Thompson ER, Boyle SE, Johnson J, et al.: Analysis of RAD51C germline mutations in high-risk breast and ovarian cancer families and ovarian cancer patients. Hum Mutat 33 (1): 95-9, 2012.
- Vuorela M, Pylkäs K, Hartikainen JM, et al.: Further evidence for the contribution of the RAD51C gene in hereditary breast and ovarian cancer susceptibility. Breast Cancer Res Treat 130 (3): 1003-10, 2011.
- Romero A, Pérez-Segura P, Tosar A, et al.: A HRM-based screening method detects RAD51C germ-line deleterious mutations in Spanish breast and ovarian cancer families. Breast Cancer Res Treat 129 (3): 939-46, 2011.
- Osorio A, Endt D, Fernández F, et al.: Predominance of pathogenic missense variants in the RAD51C gene occurring in breast and ovarian cancer families. Hum Mol Genet 21 (13): 2889-98, 2012.
- Ramus SJ, Song H, Dicks E, et al.: Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women With Ovarian Cancer. J Natl Cancer Inst 107 (11): , 2015.
- Blanco A, Gutiérrez-Enríquez S, Santamariña M, et al.: RAD51C germline mutations found in Spanish site-specific breast cancer and breast-ovarian cancer families. Breast Cancer Res Treat 147 (1): 133-43, 2014.
- Norquist BM, Harrell MI, Brady MF, et al.: Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol 2 (4): 482-90, 2016.
- Kushnir A, Laitman Y, Shimon SP, et al.: Germline mutations in RAD51C in Jewish high cancer risk families. Breast Cancer Res Treat 136 (3): 869-74, 2012.
- Wong MW, Nordfors C, Mossman D, et al.: BRIP1, PALB2, and RAD51C mutation analysis reveals their relative importance as genetic susceptibility factors for breast cancer. Breast Cancer Res Treat 127 (3): 853-9, 2011.
- Zheng Y, Zhang J, Hope K, et al.: Screening RAD51C nucleotide alterations in patients with a family history of breast and ovarian cancer. Breast Cancer Res Treat 124 (3): 857-61, 2010.
- Akbari MR, Tonin P, Foulkes WD, et al.: RAD51C germline mutations in breast and ovarian cancer patients. Breast Cancer Res 12 (4): 404, 2010.
- De Leeneer K, Van Bockstal M, De Brouwer S, et al.: Evaluation of RAD51C as cancer susceptibility gene in a large breast-ovarian cancer patient population referred for genetic testing. Breast Cancer Res Treat 133 (1): 393-8, 2012.
- Thomas G, Jacobs KB, Kraft P, et al.: A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1). Nat Genet 41 (5): 579-84, 2009.
- Figueroa JD, Garcia-Closas M, Humphreys M, et al.: Associations of common variants at 1p11.2 and 14q24.1 (RAD51L1) with breast cancer risk and heterogeneity by tumor subtype: findings from the Breast Cancer Association Consortium. Hum Mol Genet 20 (23): 4693-706, 2011.
- Osher DJ, De Leeneer K, Michils G, et al.: Mutation analysis of RAD51D in non-BRCA1/2 ovarian and breast cancer families. Br J Cancer 106 (8): 1460-3, 2012.
- Pelttari LM, Kiiski J, Nurminen R, et al.: A Finnish founder mutation in RAD51D: analysis in breast, ovarian, prostate, and colorectal cancer. J Med Genet 49 (7): 429-32, 2012.
- Golmard L, Castéra L, Krieger S, et al.: Contribution of germline deleterious variants in the RAD51 paralogs to breast and ovarian cancers. Eur J Hum Genet 25 (12): 1345-1353, 2017.
- Song H, Dicks E, Ramus SJ, et al.: Contribution of Germline Mutations in the RAD51B, RAD51C, and RAD51D Genes to Ovarian Cancer in the Population. J Clin Oncol 33 (26): 2901-7, 2015.
- Antoniou AC, Sinilnikova OM, Simard J, et al.: RAD51 135G-->C modifies breast cancer risk among BRCA2 mutation carriers: results from a combined analysis of 19 studies. Am J Hum Genet 81 (6): 1186-200, 2007.
- He XF, Su J, Zhang Y, et al.: Need for clarification of data in the recent meta-analysis about RAD51 135G>C polymorphism and breast cancer risk. Breast Cancer Res Treat 129 (2): 649-51; author reply 652-3, 2011.
- Lu W, Wang X, Lin H, et al.: Mutation screening of RAD51C in high-risk breast and ovarian cancer families. Fam Cancer 11 (3): 381-5, 2012.
- Wang WW, Spurdle AB, Kolachana P, et al.: A single nucleotide polymorphism in the 5' untranslated region of RAD51 and risk of cancer among BRCA1/2 mutation carriers. Cancer Epidemiol Biomarkers Prev 10 (9): 955-60, 2001.
- Wang Z, Dong H, Fu Y, et al.: RAD51 135G>C polymorphism contributes to breast cancer susceptibility: a meta-analysis involving 26,444 subjects. Breast Cancer Res Treat 124 (3): 765-9, 2010.
- Zhou GW, Hu J, Peng XD, et al.: RAD51 135G>C polymorphism and breast cancer risk: a meta-analysis. Breast Cancer Res Treat 125 (2): 529-35, 2011.
- Yu KD, Yang C, Fan L, et al.: RAD51 135G>C does not modify breast cancer risk in non-BRCA1/2 mutation carriers: evidence from a meta-analysis of 12 studies. Breast Cancer Res Treat 126 (2): 365-71, 2011.
Single Nucleotide Variant–Associated Cancer Risks
Polymorphisms underlying polygenic susceptibility to breast and gynecologic cancers are considered low penetrance, a term often applied to sequence variants associated with a minimal to moderate risk. This is in contrast to high-penetrance variants or alleles that are typically associated with more severe phenotypes, for example BRCA1/BRCA2 pathogenic variants leading to an autosomal dominant inheritance pattern in a family, and moderate-penetrance variants such as BRIP1, CHEK2, and RAD51C. (Refer to the
Two strategies have attempted to identify low-penetrance polymorphisms leading to breast cancer susceptibility: candidate gene and genome-wide searches. Both involve the epidemiologic case-control study design. The candidate gene approach involves selecting genes based on their known or presumed biological function, relevance to carcinogenesis or organ physiology, and then searching for or testing known genetic variants for an association with cancer risk. This strategy relies on imperfect and incomplete biological knowledge, and, despite some confirmed associations (described below), has been relatively disappointing.[
Genome-Wide Searches
In contrast to assessing candidate genes and/or alleles, GWAS involve comparing a very large set of genetic variants spread throughout the genome. The current paradigm uses sets of as many as 5 million SNVs that are chosen to capture a large portion of common variation within the genome based on the HapMap and the 1000 Genomes Project.[
Genome-wide searches are showing great promise in identifying common, low-penetrance susceptibility alleles for many complex diseases,[
Although the statistical evidence for an association between genetic variation at these loci and breast and ovarian cancer risk is overwhelming, the biologically relevant variants and the mechanism by which they lead to increased risk are unknown and will require further genetic and functional characterization. Additionally, these loci are associated with very modest risk (typically, an odds ratio [OR] <1.5), with more risk variants likely to be identified. No interaction between the SNVs and epidemiologic risk factors for breast cancer have been identified.[
More limited data are available regarding ovarian cancer risk. Three GWAS involving staged analysis of more than 10,000 cases and 13,000 controls have been carried out for ovarian cancer.[
Polygenic risk scores for breast and ovarian cancer
The collective influence of many genetic variants has more recently been evaluated using an aggregate score. In 2015, a polygenic risk score (PRS) comprising all of the known breast cancer risk genetic variants or SNVs was estimated in women of European ancestry using 41 studies in the BCAC, including more than 33,000 breast cancer cases and 33,000 controls.[
Common genomic variants associated with the development of a first primary breast cancer are also associated with the development of CBC.[
Two large studies have supported that PRSs can improve breast cancer risk stratification.[
Several studies have also examined the extent to which clinical breast cancer risk prediction models can be improved by including information on known susceptibility SNVs, and reporting improved discriminatory accuracy after inclusion of the PRS.[
A large study examined whether known reproductive and lifestyle risk factors interact with PRSs to increase breast cancer risk and did not find a multiplicative interaction with established risk factors.[
Whole-Genome and Whole-Exome Sequencing
In addition to GWAS interrogating common genetic variants, sequencing-based studies involving whole-genome or whole-exome sequencing [
References:
- Pharoah PD, Antoniou A, Bobrow M, et al.: Polygenic susceptibility to breast cancer and implications for prevention. Nat Genet 31 (1): 33-6, 2002.
- Breast Cancer Association Consortium: Commonly studied single-nucleotide polymorphisms and breast cancer: results from the Breast Cancer Association Consortium. J Natl Cancer Inst 98 (19): 1382-96, 2006.
- Dunning AM, Healey CS, Pharoah PD, et al.: A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev 8 (10): 843-54, 1999.
- Thorisson GA, Smith AV, Krishnan L, et al.: The International HapMap Project Web site. Genome Res 15 (11): 1592-3, 2005.
- Clarke L, Zheng-Bradley X, Smith R, et al.: The 1000 Genomes Project: data management and community access. Nat Methods 9 (5): 459-62, 2012.
- Evans DM, Cardon LR: Genome-wide association: a promising start to a long race. Trends Genet 22 (7): 350-4, 2006.
- Cardon LR: Genetics. Delivering new disease genes. Science 314 (5804): 1403-5, 2006.
- Chanock SJ, Manolio T, Boehnke M, et al.: Replicating genotype-phenotype associations. Nature 447 (7145): 655-60, 2007.
- Wellcome Trust Case Control Consortium: Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 (7145): 661-78, 2007.
- Easton DF, Pooley KA, Dunning AM, et al.: Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447 (7148): 1087-93, 2007.
- Stacey SN, Manolescu A, Sulem P, et al.: Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 39 (7): 865-9, 2007.
- Hunter DJ, Kraft P, Jacobs KB, et al.: A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet 39 (7): 870-4, 2007.
- Turnbull C, Ahmed S, Morrison J, et al.: Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet 42 (6): 504-7, 2010.
- Gold B, Kirchhoff T, Stefanov S, et al.: Genome-wide association study provides evidence for a breast cancer risk locus at 6q22.33. Proc Natl Acad Sci U S A 105 (11): 4340-5, 2008.
- Zheng W, Long J, Gao YT, et al.: Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet 41 (3): 324-8, 2009.
- Kibriya MG, Jasmine F, Argos M, et al.: A pilot genome-wide association study of early-onset breast cancer. Breast Cancer Res Treat 114 (3): 463-77, 2009.
- Murabito JM, Rosenberg CL, Finger D, et al.: A genome-wide association study of breast and prostate cancer in the NHLBI's Framingham Heart Study. BMC Med Genet 8 (Suppl 1): S6, 2007.
- Stacey SN, Manolescu A, Sulem P, et al.: Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 40 (6): 703-6, 2008.
- Ahmed S, Thomas G, Ghoussaini M, et al.: Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet 41 (5): 585-90, 2009.
- Reeves GK, Travis RC, Green J, et al.: Incidence of breast cancer and its subtypes in relation to individual and multiple low-penetrance genetic susceptibility loci. JAMA 304 (4): 426-34, 2010.
- Haiman CA, Chen GK, Vachon CM, et al.: A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet 43 (12): 1210-4, 2011.
- Stevens KN, Fredericksen Z, Vachon CM, et al.: 19p13.1 is a triple-negative-specific breast cancer susceptibility locus. Cancer Res 72 (7): 1795-803, 2012.
- Campa D, Kaaks R, Le Marchand L, et al.: Interactions between genetic variants and breast cancer risk factors in the breast and prostate cancer cohort consortium. J Natl Cancer Inst 103 (16): 1252-63, 2011.
- Milne RL, Gaudet MM, Spurdle AB, et al.: Assessing interactions between the associations of common genetic susceptibility variants, reproductive history and body mass index with breast cancer risk in the breast cancer association consortium: a combined case-control study. Breast Cancer Res 12 (6): R110, 2010.
- Pharoah PD, Antoniou AC, Easton DF, et al.: Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med 358 (26): 2796-803, 2008.
- Gail MH: Discriminatory accuracy from single-nucleotide polymorphisms in models to predict breast cancer risk. J Natl Cancer Inst 100 (14): 1037-41, 2008.
- Gail MH: Value of adding single-nucleotide polymorphism genotypes to a breast cancer risk model. J Natl Cancer Inst 101 (13): 959-63, 2009.
- Wacholder S, Hartge P, Prentice R, et al.: Performance of common genetic variants in breast-cancer risk models. N Engl J Med 362 (11): 986-93, 2010.
- Song H, Ramus SJ, Tyrer J, et al.: A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nat Genet 41 (9): 996-1000, 2009.
- Goode EL, Chenevix-Trench G, Song H, et al.: A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat Genet 42 (10): 874-9, 2010.
- Bolton KL, Tyrer J, Song H, et al.: Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat Genet 42 (10): 880-4, 2010.
- Mavaddat N, Pharoah PD, Michailidou K, et al.: Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst 107 (5): , 2015.
- Curtit E, Pivot X, Henriques J, et al.: Assessment of the prognostic role of a 94-single nucleotide polymorphisms risk score in early breast cancer in the SIGNAL/PHARE prospective cohort: no correlation with clinico-pathological characteristics and outcomes. Breast Cancer Res 19 (1): 98, 2017.
- Cuzick J, Brentnall AR, Segal C, et al.: Impact of a Panel of 88 Single Nucleotide Polymorphisms on the Risk of Breast Cancer in High-Risk Women: Results From Two Randomized Tamoxifen Prevention Trials. J Clin Oncol 35 (7): 743-750, 2017.
- Khera AV, Chaffin M, Aragam KG, et al.: Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 50 (9): 1219-1224, 2018.
- Kuchenbaecker KB, McGuffog L, Barrowdale D, et al.: Evaluation of Polygenic Risk Scores for Breast and Ovarian Cancer Risk Prediction in BRCA1 and BRCA2 Mutation Carriers. J Natl Cancer Inst 109 (7): , 2017.
- Li H, Feng B, Miron A, et al.: Breast cancer risk prediction using a polygenic risk score in the familial setting: a prospective study from the Breast Cancer Family Registry and kConFab. Genet Med 19 (1): 30-35, 2017.
- Li J, Ugalde-Morales E, Wen WX, et al.: Differential Burden of Rare and Common Variants on Tumor Characteristics, Survival, and Mode of Detection in Breast Cancer. Cancer Res 78 (21): 6329-6338, 2018.
- Mavaddat N, Michailidou K, Dennis J, et al.: Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am J Hum Genet 104 (1): 21-34, 2019.
- Robson ME, Reiner AS, Brooks JD, et al.: Association of Common Genetic Variants With Contralateral Breast Cancer Risk in the WECARE Study. J Natl Cancer Inst 109 (10): , 2017.
- Gao C, Polley EC, Hart SN, et al.: Risk of Breast Cancer Among Carriers of Pathogenic Variants in Breast Cancer Predisposition Genes Varies by Polygenic Risk Score. J Clin Oncol 39 (23): 2564-2573, 2021.
- Gallagher S, Hughes E, Wagner S, et al.: Association of a Polygenic Risk Score With Breast Cancer Among Women Carriers of High- and Moderate-Risk Breast Cancer Genes. JAMA Netw Open 3 (7): e208501, 2020.
- Allman R, Dite GS, Hopper JL, et al.: SNPs and breast cancer risk prediction for African American and Hispanic women. Breast Cancer Res Treat 154 (3): 583-9, 2015.
- Dite GS, MacInnis RJ, Bickerstaffe A, et al.: Breast Cancer Risk Prediction Using Clinical Models and 77 Independent Risk-Associated SNPs for Women Aged Under 50 Years: Australian Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev 25 (2): 359-65, 2016.
- Shieh Y, Hu D, Ma L, et al.: Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat 159 (3): 513-25, 2016.
- Starlard-Davenport A, Allman R, Dite GS, et al.: Validation of a genetic risk score for Arkansas women of color. PLoS One 13 (10): e0204834, 2018.
- van Veen EM, Brentnall AR, Byers H, et al.: Use of Single-Nucleotide Polymorphisms and Mammographic Density Plus Classic Risk Factors for Breast Cancer Risk Prediction. JAMA Oncol 4 (4): 476-482, 2018.
- Zhang X, Rice M, Tworoger SS, et al.: Addition of a polygenic risk score, mammographic density, and endogenous hormones to existing breast cancer risk prediction models: A nested case-control study. PLoS Med 15 (9): e1002644, 2018.
- Esserman LJ; WISDOM Study and Athena Investigators: The WISDOM Study: breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer 3: 34, 2017.
- Rudolph A, Song M, Brook MN, et al.: Joint associations of a polygenic risk score and environmental risk factors for breast cancer in the Breast Cancer Association Consortium. Int J Epidemiol 47 (2): 526-536, 2018.
- Shendure J: Next-generation human genetics. Genome Biol 12 (9): 408, 2011.
- Park DJ, Lesueur F, Nguyen-Dumont T, et al.: Rare mutations in XRCC2 increase the risk of breast cancer. Am J Hum Genet 90 (4): 734-9, 2012.
Psychosocial Issues in Hereditary Breast and Gynecologic Cancers
Introduction
Psychosocial research in the context of cancer genetic testing helps to define psychological outcomes, interpersonal and familial effects, and cultural and community responses. This type of research also identifies behavioral factors that encourage or impede screening and other health behaviors. It can enhance decision making about risk-reduction interventions, evaluate psychosocial interventions to reduce distress and/or other negative sequelae related to risk notification and genetic testing, provide data to help resolve ethical concerns, and predict the interest in testing of various groups.
Psychosocial and screening issues related to gynecologic cancers associated with Lynch syndrome are discussed in the
Uptake of Genetic Counseling and Genetic Testing
Degree of uptake of genetic counseling and genetic testing
Comparison of uptake rates among studies in which counseling and testing were offered is challenging because of differences in methodologies, including the sampling strategy used, the recruitment setting, and testing through a research protocol with high-risk cohorts or kindreds. In a systematic review of 40 studies conducted before 2002 that had assessed genetic testing utilization, uptake rates varied widely and ranged from 25% to 96%, with an average uptake rate of 59%.[
Other factors have been positively correlated with uptake of BRCA1/BRCA2 genetic testing, although these findings are not consistent across all studies. Psychological factors that have been positively correlated with testing uptake include greater cancer-specific distress and greater perceived risk of developing breast or ovarian cancer. Having more cancer-affected relatives also has been correlated with greater testing uptake.
| Study Citation | Study Population | Sample Size (N) | Uptake of GT | Predictors Associated With Uptake of GT | Comments |
|---|---|---|---|---|---|
| GC =genetic counseling; HMO = health maintenance organization. | |||||
| a Self-report as data source. | |||||
| b Medical records as data source. | |||||
| Schwartz et al. (2005)[ |
Newly diagnosed and locally untreated breast cancer patients with ≥10% risk of having aBRCA1/BRCA2 pathogenic variant a | 231 | 177/231 (77%) underwent GT | Having decided on definitive local treatment. Women who were undecided on a definitive local treatment were more likely to be tested | Testing was offered free of charge |
| 34/231 (15%) had baseline interview but declined GT | |||||
| Physician recommendation for testing. Women whose physician had recommended GT were more likely to be tested | 38/177 chose to proceed with treatment before receiving test results | ||||
| 20/231 declined baseline interview | |||||
| Kieran et al. (2007)[ |
Women who received GC between 2002 and 2004a | 250 | 88/250 (35%) underwent GT | Ability to pay for GT (entire cost or cost not covered by insurance). Nonuptake was 5.5 times more likely in women who could not afford testing | 450 women received GC for breast and ovarian cancer risk during study period. 250 women were retrospectively identified as eligible and were mailed a study questionnaire |
| 36/88 returned surveys | |||||
| Ability to recall risk estimates that were provided post-GC. Nonuptake was 15.5 times more likely in women who could not recall their risk estimates | All women had some form of insurance | ||||
| 162/250 (65%) eligible | |||||
| 65/162 returned surveys | |||||
| Susswein et al. (2008)[ |
African American women and White women with breast cancerb | 768 | 529/768 (69%) underwent GT | Race and ethnicity. African American women were less likely to be tested than White women | Sample obtained from a clinical database. Testing was offered free of charge when it was not covered by insurance. This effect for time of diagnosis was significant in the African American subgroup but not in the White subgroup |
| African American women: 77/132 (58%) underwent GT | |||||
| Recent diagnosis. African American women who were recently diagnosed were more likely to be tested | |||||
| White women: 452/636 (71%) underwent GT | |||||
| Olaya et al. (2009)[ |
Patients referred for GT between 2001 and 2008b | 213 | 111/213 (52%) underwent GT | Personal history of breast cancer. Having a personal history was associated with 3 times greater odds of being tested | Insurance coverage for testing was available for 91.1% (175/213) of patients. Of those who had coverage for GT, 51.4% underwent testing and 48.6% did not. Of those without coverage, 41.2% had GT and 58.9% did not |
| 102/213 (48%) declined GT | Higher level of education. Those with a high school education or less had one-third the odds of being tested, compared with those with at least some college | ||||
| Levy et al. (2010)[ |
Women aged 20–40 y with newly diagnosed early-onset breast cancer.b | 1,474 | 446/1,474 (30%) underwent GT | Race and ethnicity. Women of Jewish ethnicity were 3 times more likely to be tested than non-Jewish White women. African American and Hispanic women were significantly less likely to receive testing than non-Jewish White women | Sample obtained from a national database of commercially insured individuals |
| Jewish women: 18/32 (56%) underwent GT | Home location. Women living in the south were more likely to be tested than women living in the northeast | ||||
| African American women: 10/82 (12%) underwent GT | Insurance type. Women with point-of-service plans were more likely to be tested than women with HMO plans | ||||
| Recent diagnosis. Women diagnosed in 2007 were 3.8 times more likely to be tested than women diagnosed in 2004 | |||||
Several studies conducted in non-U.S. settings have examined the uptake of genetic testing.[
Factors influencing uptake of genetic counseling and genetic testing
In reviews that have examined the cumulative evidence concerning the predictors of uptake of BRCA1/BRCA2 genetic testing, important predictors of testing uptake include older age, Ashkenazi Jewish (AJ) heritage, unmarried status, a personal history of breast cancer, and a family history of breast cancer. Studies recruiting participants in hospital settings had significantly higher recruitment rates than did studies recruiting participants in community settings. Studies that required an immediate decision to test, rather than allowing delayed decision making, tended to report higher uptake rates.[
In a review of racial and ethnic differences that affect the uptake of BRCA1/BRCA2 testing, intention to undergo genetic testing in African American women was related to having at least one FDR with breast cancer or ovarian cancer, higher perceived risk of being a carrier, and less anticipatory guilt about the possibility of being a gene carrier.[
Reasons cited for following through with testing included a desire to learn about a child's risk, to feel relief from uncertainty, to inform screening or risk-reducing surgery decisions, and to inform important life decisions such as marriage and childbearing.[
Physician recommendation may be another motivator for testing. In a retrospective study of 335 women considering genetic testing, 77% reported that they wanted the opinion of a genetics physician about whether they should be tested, and 49% wanted the opinion of their primary care provider.[
The uptake of BRCA testing to inform surgical treatment decisions when offered appears to be high in research cohorts;[
Insurance coverage
Insurance coverage is an important consideration for individuals deciding whether to undergo genetic testing. (Refer to the
Uptake of genetic counseling and genetic testing in diverse populations
Degree of uptake of genetic counseling and genetic testing in diverse populations
There are limited data on uptake of genetic counseling and testing among non-White populations, and further research will be needed to define factors influencing uptake in these populations.[
Notably, the racial differences observed in these studies do not appear to be explained by factors related to cost, access to care, risk factors for carrying a BRCA1 or BRCA2 pathogenic variant, or differences in psychosocial factors, including risk perceptions, worry, or attitudes toward testing.
Factors influencing uptake of genetic counseling and genetic testing in diverse populations
Several studies have examined uptake or "acceptance" of BRCA testing among African American individuals enrolled in genetic research programs. Among study enrollees from an African American kindred in Utah, 83% underwent BRCA1 testing.[
Work examining attitudes toward breast cancer genetic testing in Latino and African American populations indicates limited knowledge and awareness about testing but a generally receptive view once they are informed; in comparison with White populations, Latino and African American populations have relatively more concerns about testing.
For example, in a qualitative study with 51 Latino individuals unselected for risk status, important findings included the fact that participants were highly interested in genetic testing for inherited cancer susceptibility, despite very limited knowledge about genetics. One important barrier involved secrecy or embarrassment about family discussions of cancer and genetics, which could be addressed in intervention strategies.[
A telephone survey of 314 patients from an inner-city network of Pittsburgh, Pennsylvania, health centers, 50% of whom were African American, found that most participants (57%) (both African American and White participants) felt that genetic testing to evaluate disease risk was a good idea; however, more African American participants than White participants thought that genetic testing would lead to racial discrimination (37% vs. 22%, respectively) and that genetics research was unethical and tampered with nature (20% vs. 11%, respectively).[
In a sample of 146 African American women meeting criteria for BRCA1/BRCA2 pathogenic variant testing, women born outside the United States reported higher levels of anticipated negative emotional reactions (e.g., fear, hopelessness, and lack of confidence that they could emotionally handle testing). Higher levels of breast cancer–specific distress were associated with anticipated negative emotional reactions, confidentiality concerns, and anticipated guilt regarding the family impact of breast cancer genetic testing.[
There are racial differences in provider discussion and patient uptake of genetic testing for variants in BRCA1/BRCA2. A study of women aged 18 to 64 years and diagnosed with invasive breast cancer between 2007 and 2009 found that, even after adjusting for pathogenic variant risk, African American women were less likely to report having received a physician recommendation for genetic testing. There was no difference across all races in concerns that BRCA1/BRCA2 testing was too expensive and only minimal differences in testing attitudes or insurance concerns were found, none of which influenced testing uptake.[
Factors associated with declining genetic counseling and testing
There is evidence that primary reasons for declining testing involves being childless, which reduces any family motivations for testing; and concerns about the negative ramifications of testing, including difficulty retaining insurance or concerns about personal health.
Limited data are available about the characteristics of at-risk individuals who decline to be tested or have never been tested. It is difficult to access samples of test decliners because they may be reluctant to participate in research studies. Studies of genetic testing uptake are difficult to compare because people may decline at different points and with different amounts of pretest education and counseling. One study found that 43% of affected and unaffected individuals from hereditary breast/ovarian cancer families who completed a baseline interview regarding testing declined to be tested. Most individuals who declined testing chose not to participate in educational sessions. Decliners were more likely to be male and be unmarried and have fewer relatives with breast cancer. Decliners who had high levels of cancer-related stress had higher levels of depression. Decliners lost to follow-up were significantly more likely to be affected with cancer.[
Another study looked at a small number (n = 13) of women decliners who carried a 25% to 50% probability of harboring a BRCA pathogenic variant; these nontested women were more likely to be childless and to have higher levels of education. This study showed that most women decided not to undergo the test after serious deliberation about the risks and benefits. Satisfaction with frequent surveillance was given as one reason for nontesting by most of these women.[
A third study evaluated characteristics of 34 individuals who declined BRCA1/BRCA2 testing in a large multicenter study in the United Kingdom. Decliners were younger than a national sample of test acceptors, and female decliners had lower mean scores on a measure of cancer worry. Although 78% of test decliners/deferrers felt that their health was at risk, they reported that learning about their BRCA1/BRCA2 pathogenic variant status would cause them to worry about the following:
- Their children's health (76%).
- Their life insurance (60%).
- Their own health (56%).
- Loss of their job (5%).
- Receiving less screening if they did not carry a BRCA1/BRCA2 pathogenic variant (62%).
Apprehension about the impact of the test result was a more important factor in the decision to decline testing than were concrete burdens such as time required to travel to a genetics clinic and time spent away from work, family, and social obligations.[
Genetic counseling and testing in children
Testing for BRCA1/BRCA2 pathogenic variants has been almost universally limited to adults older than 18 years. The risks of testing children for adult-onset disorders, such as breast and ovarian cancers, as inferred from developmental data on children's medical understanding and ability to provide informed consent, have been outlined in several reports.[
Studies suggest that individuals who have undergone BRCA1/BRCA2 genetic testing or who are adult offspring of individuals who have had testing are generally not in favor of testing minors.[
No data exist on the testing of children for BRCA1/BRCA2 pathogenic variants, although some researchers believe it is necessary to test the validity of assumptions underlying the general prohibition of testing children for genetic variants associated with breast and ovarian cancers and other adult-onset diseases.[
What People Bring to Genetic Testing: Impact of Risk Perception, Health Beliefs, and Personality Characteristics
The emerging literature in this area suggests that risk perceptions, health beliefs, psychological status, and personality characteristics are important factors in decision making about breast/ovarian cancer genetic testing. Many women presenting at academic centers for BRCA1/BRCA2 testing arrive with a strong belief that they have a pathogenic variant, having decided they want genetic testing, but possessing little information about the risks or limitations of testing.[
A general tendency to overestimate inherited risk of breast and ovarian cancer has been noted in at-risk populations,[
Women appear to be the prime communicators within families about the family history of breast cancer.[
The accuracy of reported family history of breast or ovarian cancer varies; some studies found levels of accuracy above 90%,[
Targeted written,[
Genetic Counseling for Hereditary Predisposition to Breast Cancer
Counseling for breast cancer risk typically involves individuals with family histories that are potentially attributable to BRCA1 or BRCA2. It also, however, may include individuals with family histories of Li-Fraumeni syndrome, ataxia-telangiectasia, Cowden syndrome, or Peutz-Jeghers syndrome.[
Management strategies for carriers may involve decisions about the nature, frequency, and timing of screening and surveillance procedures, chemoprevention, risk-reducing surgery, and use of hormone replacement therapy (HRT). The utilization of breast conservation and radiation as cancer therapy for women who are carriers may be influenced by knowledge of pathogenic variant status. (Refer to the
Counseling also includes consideration of related psychosocial concerns and discussion of planned family communication and the responsibility to warn other family members about the possibility of having an increased risk of breast, ovarian, and other cancers. Data suggest that individual responses to being tested as adults are influenced by the results status of other family members.[
Published descriptions of counseling programs for BRCA1 (and subsequently for BRCA2) testing include strategies for gathering a family history, assessing eligibility for testing, communicating the considerable volume of relevant information about breast/ovarian cancer genetics and associated medical and psychosocial risks and benefits, and discussion of specialized ethical considerations about confidentiality and family communication.[
Many of the psychosocial outcome studies involve specialized, highly selected research populations, some of which were utilized to map and clone BRCA1 and BRCA2. One such example is K2082, an extensively studied kindred of more than 800 members of a Utah Mormon family in which a BRCA1 pathogenic variant accounts for the observed increased rates of breast and ovarian cancer. A study of the understanding that members of this kindred have about breast/ovarian cancer genetics found that, even in breast cancer research populations, there was incomplete knowledge about associated risks of colon and prostate cancer, the existence of options for risk-reducing mastectomy (RRM) and risk-reducing salpingo-oophorectomy (RRSO), and the complexity of existing psychosocial risks.[
Emotional Outcomes
Although there were initial concerns about the possibility of adverse emotional consequences from BRCA testing, most studies conducted over the years have shown low levels of psychological distress among both carriers and noncarriers, particularly over the longer term.[
Several studies have reported on emotional outcomes over longer follow-up periods (i.e., greater than 12 months after disclosure) than those reported in the meta-analysis described above.[
Although most studies have reported that a positive BRCA test result has a relatively minimal impact on psychological distress, many of these studies were conducted among families with a strong family history of breast or ovarian cancer who underwent extensive pretest genetic counseling. Therefore, emotional responses may not generalize to individuals who test under different contexts. For example, individuals who are tested with population BRCA screening may not have a family history of cancer.[
For example, in a Canadian study of 2,080 Jewish women who participated in a population-based genetic screening study to test for three BRCA pathogenic variants common in families of Jewish heritage, women were not offered in-person genetic counseling but were given a pamphlet on genetic testing for BRCA1/BRCA2 before they provided a DNA sample. One year after genetic testing, women who were positive for a pathogenic variant (n = 18) showed significant increases in cancer-specific distress, whereas no changes in distress were observed among women who were negative for a pathogenic variant.[
Similarly, the impact of direct-to-consumer (DTC) BRCA testing through commercial companies requires further evaluation. Case studies have reported adverse emotional responses after receipt of a positive BRCA result from DTC genetic testing, suggesting the need for further evaluation of the emotional outcomes of women undergoing genetic testing through commercial companies.[
Despite evidence of a short-term increase in distress after the receipt of genetic testing results, any adverse responses to a positive carrier status dissipate within 12 months.[
Emotional outcomes in newly diagnosed breast cancer patients
It is increasingly common for women with breast cancer to pursue genetic counseling and testing at the time of diagnosis to assist with treatment decision making. (Refer to the
Family Effects
Family communication about genetic testing and hereditary risk
Family communication about genetic testing for cancer susceptibility, and specifically about the results of BRCA1/BRCA2 genetic testing, is complex. Age and gender appear to be important variables in the disclosure of genetic test results. Studies have documented that older women were more likely to disclose genetic test results (especially to their daughters) than younger women.[
Family communication of BRCA1/BRCA2 test results to relatives is another factor affecting participation in testing. There have been more studies of communication with FDRs and SDRs than with more distant family members. Studies have investigated the process and content of communication among sisters about BRCA1/BRCA2 test results.[
Among relatives with whom genetic test results were not discussed, the most important reason given was that the affected women were not close to their relatives [
A study that evaluated communication of test results to FDRs at 4 months postdisclosure found that participants were more likely to inform brothers of their results if the BRCA pathogenic variant was inherited through the paternal line.[
A few in-depth qualitative studies have looked at issues associated with family communication about genetic testing. Although the findings from these studies may not be generalizable to the larger population of at-risk individuals, they illustrate the complexity of issues involved in conveying hereditary cancer risk information in families.[
A study of 31 mothers with a documented BRCA pathogenic variant explored patterns of dissemination to children.[
A longitudinal study of 153 women self-referred for genetic testing for BRCA1 and BRCA2 pathogenic variants and 118 of their partners evaluated communication about genetic testing and distress before testing and at 6 months posttesting.[
A study of 561 FDRs of women who had undergone BRCA1/BRCA2 genetic testing found that 22% of FDRs did not recall being informed of the genetic test results despite the women reporting that the results had been shared.[
There is a small but growing body of literature regarding psychological effects in men who have a family history of breast cancer and who are considering or have had BRCA testing. A qualitative study of 22 men from 16 high-risk families in Ireland revealed that more men in the study with daughters were tested than men without daughters. These men reported little communication with relatives about the illness, with some men reporting being excluded from discussion about cancer among female family members. Some men in the study also reported actively avoiding open discussion with daughters and other relatives.[
Family functioning
One study assessed 212 individuals from 13 families with HBOC who received genetic counseling and were offered BRCA1/BRCA2 testing for documented pathogenic variants in the family. Individuals who were not tested were found 6 to 9 months later to have significantly greater increases in family expressiveness and cohesiveness compared with those who were tested. Individuals who were randomly assigned to a client-centered versus problem-solving genetic counseling intervention had a significantly greater reduction in conflict, regardless of the test decision.[
Partners of high-risk women
Many studies have looked at the psychological effects in women of having a high risk of developing cancer, either on the basis of carrying a BRCA1/BRCA2 pathogenic variant or having a strong family history of cancer. Some studies have also examined the effects on the partners of such women.
A Canadian study assessed 59 spouses of women found to have a BRCA1/BRCA2 pathogenic variant. All were supportive of their spouses' decision to undergo genetic testing and 17% wished they had been more involved in the genetic testing process. Spouses who reported that genetic testing had no impact on their relationship had long-term relationships (mean duration 27 years). Forty-six percent of spouses reported that their major concern was of their partner dying of cancer. Nineteen percent were concerned their spouse would develop cancer and 14% were concerned their children would also be carriers of BRCA1/BRCA2 pathogenic variants.[
In a U.S. study, 118 partners of women who underwent genetic testing for pathogenic variants in BRCA1 and BRCA2 completed a survey before testing and then again 6 months after result disclosure. At 6 months, only 10 partners reported that they had not been told of the test result. Ninety-one percent reported that the testing had not caused strain on their relationship. Partners who were comfortable sharing concerns before testing experienced less distress after testing. Protective buffering was not found to impact distress levels of partners.[
An Australian study of 95 unaffected women at high risk of developing breast and/or ovarian cancer (13 carriers of pathogenic variants and 82 with unknown variant status) and their partners showed that although the majority of male partners had distress levels comparable to a normative population sample, 10% had significant levels of distress that indicated the need for further clinical intervention. Men with a high monitoring coping style and greater perceived breast cancer risk for their wives reported higher levels of distress. Open communication between the men and their partners and the occurrence of a cancer-related event in the wife's family in the last year were associated with lower distress levels. When men were asked what kind of information and support they would like for themselves and their partners, 57.9% reported that they would like more information about breast and ovarian cancer, and 32.6% said they would like more support in dealing with their partner's risk. Twenty-five percent of men had suggestions on how to improve services for partners of high-risk women, including strategies on how to best support their partner, greater encouragement from health care professionals to attend appointments, and meeting with other partners.[
A review of this literature reported that the BRCA testing process may be distressing for male partners, particularly for those with spouses identified as carriers. Male partner distress appears to be associated with their beliefs about the woman's breast cancer risk, lack of couple communication, and feelings of alienation from the testing process.[
At-risk males
A review of the literature on the experiences of males in families with a known BRCA1 and BRCA2 pathogenic variant reported that while the data are limited, men from variant-positive families are less likely than females to participate in communication regarding genetics at every level, including the counseling and testing process. Men are less likely to be informed of genetic test results received by female relatives, and most men from these families do not pursue their own genetic testing.[
A study of Dutch men at increased risk of having inherited a BRCA1 pathogenic variant reported a tendency for the men to deny or minimize the emotional effects of their risk status, and to focus on medical implications for their female relatives. Men in these families, however, also reported considerable distress in relation to their female relatives.[
A multicenter U.K. cohort study examined prospective outcomes of BRCA1/BRCA2 testing in 193 individuals, of which 20% were men aged 28 to 86 years. Men's distress levels were low, did not differ among carriers and noncarriers, and did not change from baseline (before genetic testing) to the 3-year follow-up. Twenty-two percent of male carriers of pathogenic variants received colorectal cancer screening and 44% received prostate cancer screening;[
Children
Several studies have explored communication of BRCA test results to at-risk children. Across all studies, the rate of disclosure to children ranging in age from 4 to 25 years is approximately 50%.[
Several studies have also looked at the timing of disclosure to children after parents receive their test results. Although the majority of children were told within a week to several months after results disclosure,[
In one study, participants who told children younger than 13 years about their carrier status had increased distress, and those who did not tell their young children experienced a slight decrease in distress. Communication with young children was found to be influenced by developmental variables such as age and style of parent/child communication.[
One study looked at the reaction of children to results disclosure or the effect on the parent-child relationship of communicating the results.[
Interestingly, a large multicenter study of 869 mother-daughter pairs (the daughters were aged 6 to 13 y) found that girls with a family history of breast cancer or a familial BRCA1/BRCA2 pathogenic variant compared with those without such family histories had better psychosocial adjustment by maternal report.[
Another study of 187 mothers undergoing BRCA1/BRCA2 testing evaluated their need for resources to prepare for a facilitated conversation about sharing their BRCA1/BRCA2 testing results with their children. Seventy-eight percent of mothers were interested in three or more resources, including literature (93%), family counseling (86%), talking to prior participants (79%), and support groups (54%).[
Testing for BRCA1/BRCA2 has been almost universally limited to adults older than 18 years. The risks of testing children for adult-onset disorders (such as breast and ovarian cancer), as inferred from developmental data on children's medical understanding and ability to provide informed consent, have been outlined in several reports.[
Prenatal diagnosis and preimplantation genetic testing
The possibility of transmitting a pathogenic variant to a child may pose a concern to families affected by HBOC,[
In the United States, a series of studies has evaluated awareness, interest (e.g., would consider using PGT), and attitudes related to PGT among members of Facing Our Risk of Cancer Empowered (FORCE), an advocacy organization focused on individuals at increased risk of HBOC.[
It is unknown whether the attitudes of FORCE members toward PGT are representative of the majority of BRCA carriers. A cross-sectional study of 1,081 BRCA carriers, 65% of whom were recruited through FORCE and the remainder by the University of Pennsylvania, revealed that a majority of carriers were in favor of offering PGT and prenatal diagnosis to carriers (59% for PGT and 55.5% for prenatal diagnosis).[
The U.K. Human Fertilization and Embryology authority has approved the use of PGT for HBOC. In a sample of 102 women with a BRCA pathogenic variant, most were supportive of PGT but only 38% of the women who had completed their families would consider it for themselves had PGT been available, and only 14% of women who were contemplating a future pregnancy would consider PGT.[
In France, couples who obtain authorization from a multidisciplinary prenatal diagnosis team may access PGT free of charge as a benefit of their national health care system. However, no BRCA carriers have been authorized to use PGT. In a national study of 490 unaffected carriers of BRCA pathogenic variants of childbearing age (women aged 18–49 y; men aged 18–69 y), 16% stated that BRCA test results had altered their ongoing plans for childbearing.[
A small (N = 25) qualitative study of women of reproductive age positive for a BRCA pathogenic variant who underwent genetic testing before having children evaluated how their BRCA status influenced their attitudes about reproductive genetic testing (both PGT and prenatal diagnosis) and decisions about having children.[
One study has examined these issues among high-risk men recruited from FORCE and Craigslist (a bulletin board website) (N = 228).[
Cultural/Community Effects
The recognition that BRCA1/BRCA2 pathogenic variants are prevalent, not only in breast/ovarian cancer families but also in some ethnic groups,[
A growing literature on the unique factors influencing a variety of cultural subgroups suggests the importance of developing culturally specific genetic counseling and educational approaches.[
Ethical Concerns
The human implications of the ethical issues raised by the advent of genetic testing for breast/ovarian cancer susceptibility are described in case studies,[
Studies have shown that 62% of studied family members were aware of the family history and that 88% of hereditary breast/ovarian cancer family members surveyed have significant concerns about privacy and confidentiality. Concern about cancer in third-degree relatives, or relatives further removed, did not differ from concern about cancer in the proband's FDRs or SDRs.[
Psychosocial Aspects of Cancer Risk Management for Hereditary Breast and Gynecologic Cancers
Decision aids for individuals considering risk management options for hereditary breast and gynecologic cancers
A systematic review of decision aids for BRCA1/BRCA2 carriers suggested that these tools are beneficial during the decision-making process.[
A telephone-based intervention study used integrated motivational interviewing techniques and was led by genetic counselors.[
Uptake of cancer risk management options
Several studies have examined uptake and adherence to cancer risk management options in individuals who have had genetic counseling and testing for BRCA1 and BRCA2 pathogenic variants. Outcomes vary across studies and include the following: 1) uptake/adherence to screening options (mammography, magnetic resonance imaging [MRI]) and 2) selection of risk-reducing options (i.e., risk-reducing mastectomy [RRM] and/or RRSO).
Most studies reported outcomes by pathogenic variant carrier status. Follow-up time after participants received their genetic test results also varied across studies, ranging from 12 months to several years. The Hereditary Breast Cancer Clinical Study Group assessed uptake of cancer risk management options in 6,223 women with a BRCA1/BRCA2 pathogenic variant.[
Mammography
- In a personal communication, the first author shared that mammography uptake was defined as participants' self-reporting of mammography screenings after they received their genetic test results (K. Metcalfe, PhD, email, May 30, 2023).[
218 ] Mammography uptake was 82%, but this value diminished over time. The authors suggested that this decrease in mammography uptake may have been due to an increase in breast MRI uptake in participating countries, like Poland, where use of MRI exceeds the use of mammography.
MRI
- In a personal communication, the first author shared that MRI uptake was defined as participants' self-reporting of screening MRIs after they received their genetic test results (K. Metcalfe, PhD, email, May 30, 2023).[
218 ] The rate of screening MRI increased in women tested from Canada, Poland, and the United States from 2009 and onward (81.3%) when compared with women who tested prior to 2009 (69.5%). Overall, 72.8% of women in this study reported receiving a breast MRI in the past year.
Risk-reducing mastectomy (RRM)
- Uptake of RRM in women without a history of breast cancer was 27.8%, with RRM occurring at a mean age of 41.8 years (range, 19–78 y).
- The rate of RRM uptake was the highest in the United States (49.9%).
Risk-reducing bilateral salpingo-oophorectomy (RRSO)
- Uptake of RRSO varied slightly by gene, with 62.8% of BRCA1 carriers and 69.7% of BRCA2 carriers receiving RRSO.
- Uptake of RRSO was 70.7% in women with personal histories of breast cancer.
- For women who chose RRSO, the age of surgery also varied by gene. In BRCA1 carriers, 7.2% had RRSO performed before age 35 years, whereas in BRCA2 carriers, 37.8% had RRSO performed before age 45 years.
Chemoprevention
- Use of tamoxifen or raloxifene for risk reduction in women without histories of breast cancer was 6.3% (n = 155/2463).
- Chemoprevention use was highest in the United States (15%), with use occurring in 12.7% of BRCA1 carriers and in 17.4% of BRCA2 carriers.
In summary, breast cancer screening with mammogram in BRCA1/BRCA2 carriers may be suboptimal and can diminish over time.[
Women were more likely to undergo RRM when testing from 2009 and onward (30.3%) when compared with those who tested prior to 2009 (26.9%; P = .04).[
Uptake of RRSO was associated with increasing age, White race, having children, a family history of ovarian cancer, a personal history of mastectomy, higher perceived risk of ovarian cancer, and higher perceived benefit of RRSO.[
BRCA testing, when offered to women newly diagnosed with breast cancer, has been shown to influence surgical decision making in that carriers are more likely to opt for bilateral mastectomy compared with noncarriers.[
A study conducted from 2006 to 2014 in 11 U.S. academic and community centers of 897 women, aged 40 years and younger at breast cancer diagnosis, found that rates of BRCA genetic testing have increased over time.[
A systematic review of risk-reducing salpingectomy with delayed oophorectomy (RRDSO) found that the avoidance of surgical menopause, preservation of fertility, concerns about sexual functioning, a family history of breast cancer, and an interest to avoid HRT were all related to being more likely to accept this option.[
Cancer screening and risk-reducing behaviors
Data are now emerging regarding uptake and adherence to cancer risk management recommendations such as screening and risk-reducing interventions. Cancer screening adherence and risk-reduction behaviors as defined by the National Comprehensive Cancer Network Guidelines were assessed in a cross-sectional study of 214 women with a personal history (n = 134) or family history (n = 80) of breast or ovarian cancer. Among unaffected women older than 40 years, 10% had not had a mammogram or clinical breast examination (CBE) in the previous year and 46% did not practice breast self-examination (BSE). Among women previously affected with breast or ovarian cancer, 21% had not had a mammogram, 32% had not had a CBE, and 39% did not practice BSE.[
Three hundred and twelve women who were counseled and tested for BRCA pathogenic variants between 1997 and 2005 responded to a survey regarding their perception of genetic testing for HBOC. The survey included questions on risk reduction options, including screening and risk-reducing surgeries. Two hundred and seventeen (70%) of the women had been diagnosed with breast cancer, and 86 (28%) tested positive for a pathogenic variant in either the BRCA1 or BRCA2 gene. None of the BRCA-positive women agreed that mammograms are difficult procedures because of the discomfort, while 11 (5.4%) of the BRCA-negative women did agree with this statement. Both groups (BRCA-positive and BRCA-negative) agreed that risk-reducing surgeries provide the best means for lowering cancer risk and worry, and most patients in both groups expressed the belief that RRM is not too drastic, too scary, or too disfiguring.[
A prospective study from the United Kingdom examined the psychological impact of mammographic screening in 1,286 women aged 35 to 49 years who have a family history of breast cancer and were participants in a multicenter screening program. Mammographic abnormalities that required additional evaluation were detected in 112 women. These women, however, did not show a statistically significant increase in cancer worry or negative psychological consequences as a result of these findings. The 1,174 women who had no mammographic abnormality detected experienced a decrease in cancer worry and a decrease in negative psychological consequences compared with baseline after receipt of their results. At 6 months, the entire cohort had experienced a decrease in measures of cancer worry and psychological consequences of breast screening.[
A qualitative study explored health care professionals' views regarding the provision of information about health protective behaviors (e.g., exercise and diet). Seven medical specialists and ten genetic counselors were interviewed during a focus group or individually. The study reported wide variation in the content and extent of information provided about health-protective behaviors and in general, participants did not consider it their role to promote such behaviors in the context of a genetic counseling session. There was agreement, however, about the need to form consensus about provision of such information both within and across risk assessment clinics.[
Not all studies specify whether screening uptake rates fall within recommended guidelines for the targeted population or the specific clinical scenario, nor do they report on other variables that may influence cancer screening recommendations. For example, women who have a history of atypical ductal hyperplasia would be advised to follow screening recommendations that may differ from those of the general population.
Psychosocial Outcome Studies
Psychosocial outcomes associated with risk-reducing mastectomy (RRM)
A prospective study conducted in the Netherlands found that among 26 carriers of BRCA1/BRCA2 pathogenic variants, the 14 women who chose mastectomy had higher distress both before test result disclosure and 6 and 12 months later, compared with the 12 carriers who chose surveillance and compared with 53 women negative for a pathogenic variant. Overall, however, anxiety declined in women undergoing RRM; at 1 year, their anxiety scores were closer to those of women choosing surveillance and to the scores of women negative for a pathogenic variant.[
Mixed psychosocial outcomes were reported in a follow-up study (mean, 14 y) of 609 women who received RRM at the Mayo Clinic. Seventy percent were satisfied with RRM, 11% were neutral, and 19% were dissatisfied. Eighteen percent believed that if they had the choice to make again, they probably or definitely would not have an RRM. About three-quarters said their worry about cancer was diminished by surgery. One-half reported no change in their satisfaction with body image; 16% reported improved body image after surgery. Thirty-six percent said they were dissatisfied with their body image after RRM. About one-quarter of the women reported adverse impact of RRM on their sexual relationships and sense of femininity, and 18% had diminished self-esteem. Factors most strongly associated with satisfaction with RRM were postsurgical satisfaction with appearance, reduced stress, no reconstruction or lack of problems with implants, and no change or improvement in sexual relationships. Women who cited physician advice as the primary reason for choosing RRM tended to be dissatisfied after RRM.[
A study of 60 healthy women who underwent RRM measured levels of satisfaction, body image, sexual functioning, intrusion and avoidance, and current psychological status at a mean of 4 years and 4 months postsurgery. Of this group, 76.7% had either a strong family history (21.7%) or carried a BRCA1 or BRCA2 pathogenic variant (55%). Overall, 97% of the women surveyed were either satisfied (17%) or extremely satisfied (80%) with their decision to have RRM, and all but one participant would recommend this procedure to other women. Most women (66.7%) reported that surgery had no impact on their sexual life, although 31.7% reported a worsening sexual life, and 76.6% reported either no change in body image or an improvement in body image, regardless of whether reconstruction was performed. Worsening self-image was reported by 23.3% of women after surgery. Women's mean distress levels after surgery were only slightly above normal levels, although those women who continued to perceive their postsurgery breast cancer risk as high had higher mean levels of global and cancer-related distress than those who perceived their risk as low. Additionally, carriers of BRCA1 and BRCA2 pathogenic variants and women with a strong family history of breast and/or ovarian cancer had higher mean levels of cancer-related distress than women with a limited family history.[
Very little is known about how the results of genetic testing affect treatment decisions at the time of cancer diagnosis. Two studies explored genetic counseling and BRCA1/BRCA2 genetic testing at the time of breast cancer diagnosis.[
A prospective study from the Netherlands evaluated long-term psychological outcomes of offering women with breast cancer genetic counseling and, if indicated, genetic testing at the onset of breast radiation for treatment of their primary breast cancer. Of those who were approached for counseling, some underwent genetic testing and chose to receive their result (n = 58), some were approached but did not fulfill referral criteria (n = 118), and some declined the option of counseling/testing (n = 44). Another subset of women undergoing radiation therapy was not approached for counseling (n = 182) but was followed using the same measures. Psychological distress was measured at baseline and at 4, 11, 27, and 43 weeks after initial consultation for radiation therapy. No differences were detected in general anxiety, depression or breast cancer–specific distress across all four groups.[
A retrospective questionnaire study of 583 women with a personal and family history of breast cancer and who underwent contralateral RRM between 1960 and 1993 measured overall satisfaction after mastectomy and factors influencing satisfaction and dissatisfaction with this procedure.[
A retrospective survey of 137 BRCA carriers examined the psychosocial impact of preserving the nipple-areolar complex (NAC) in women with bilateral RRM.[
Another study compared long-term quality-of-life outcomes in 195 women after bilateral RRM performed between 1979 and 1999 versus 117 women at high risk of breast cancer opting for screening. No statistically significant differences were detected between the groups for psychosocial outcomes. Eighty-four percent of those opting for surgery reported satisfaction with their decision. Sixty-one percent of women from both the surgery and screening groups reported being very much or quite a bit contented with their quality of life.[
In a study of psychosocial outcomes associated with RRM and immediate reconstruction, 61 high-risk women (27 carriers of pathogenic variants, others with high-risk family history), 31 of whom had a prior history of breast cancer, were evaluated on average 3 to 4 years after surgery.[
A qualitative study examining material on the
In contrast, another study examined long-term psychosocial outcomes in 684 women who had had bilateral or contralateral RRM on average 9 years before assessment.[
In a qualitative study of 108 women who underwent or were considering RRM, more than half of those who had RRM felt that presurgical consultation with a psychologist was advisable; nearly two-thirds thought that postsurgical consultation was also appropriate. All of the women who were considering RRM believed that psychological consultation before surgery would facilitate decision-making.[
Psychosocial outcomes associated with risk-reducing salpingo-oophorectomy (RRSO)
Prior studies on the impact of RRSO suggest some variability in outcomes based on menopausal status:
- A Canadian prospective study examined the impact of RRSO on menopausal symptoms and sexual functioning in a sample of 140 women with known BRCA1/BRCA2 pathogenic variants.[
250 ] This study compared outcomes before surgery and after surgery (an average of 3.5 y later). RRSO was associated with an increase in menopausal symptoms and a decrease in reported sexual functioning in premenopausal women (n = 93). However, differences in overall quality of life (QOL) were not reported in this group. HRT reduced the adverse effects associated with RRSO but did not fully eliminate them. In postmenopausal women (n = 47), RRSO resulted in an increase in physical symptoms (i.e., insomnia and fatigue) and decreases in both sexual functioning and overall QOL. - A cross-sectional study analyzed 244 women with BRCA1/BRCA2 pathogenic variants who were aged 40 years or older.[
251 ]BRCA1/BRCA2 carriers who did not undergo RRSO had more cancer worry than women who had undergone RRSO, but fewer menopausal symptoms. Those who underwent RRSO and those who did not had similar levels of sexual activity and functioning. Comparing women with pre- vs. postmenopausal RRSO, premenopausal women were more likely to be sexually active, to report menopausal symptoms, and to have depression than postmenopausal women. However, premenopausal and postmenopausal women had similar levels of sexual functioning. For more information, see theRisk-reducing salpingo-oophorectomy for ovarian cancer risk reduction section in BRCA1 and BRCA2: Cancer Risks and Management. - A cross-sectional study of 641 women (66% of whom were BRCA1/BRCA2 carriers) who underwent RRSO before or after menopause found no long-term association between premenopausal RRSO and measures of cognitive function.[
252 ]
Behavioral Outcomes
A study [
While motivations cited for pursuing genetic testing often include the expectation that carriers of pathogenic variants will be more compliant with breast and/or ovarian screening recommendations,[
This is a critical issue not only for women testing positive, but also for adherence to screening for those testing negative and those who have received indeterminate results or choose not to receive their results. It is possible that adherence actually diminishes with a decrease in the perceived risk that may result from a negative genetic test result.
In addition, while there is still some question regarding the link between cancer-related worry and breast cancer screening behavior, accumulating evidence appears to support a linear rather than a curvilinear relationship. That is, for some time, the data were not consistent; some data supported the hypothesis that mild-to-moderate worry may increase adherence, while excessive worry may actually decrease the utilization of recommended screening practices. Other reports support the notion that a linear relationship is more likely; that is, more worry increases adherence to screening recommendations. Few studies, however, have followed women to assess their health behaviors after genetic testing. Thus, a negative test result leading to decreased worry could theoretically result in decreased screening adherence. A large study found that patient compliance with screening practices was not related to general or screening-specific anxiety—with the exception of BSE, for which compliance is negatively associated with procedure-specific anxiety.[
Further complicating this area of research are issues such as the baseline rate of mammography adherence among women older than 40 or 50 years before genetic testing. More specifically, the ability to note a significant difference in adherence on this measure may be affected by the high adherence rate to this screening behavior before genetic testing by women undergoing such testing. It may be easier to find significant changes in mammography use among women with a family history of breast cancer who test positive. Finally, adherence over time will likely be affected by how women undergoing genetic testing and their caregivers perceive the efficacy of many of the screening options in question, such as mammography for younger women, BSE, and ovarian cancer screening (periodic vaginal ultrasound and serum CA-125 measurements), along with the value of preventive interventions.
The issue of screening decision-making and adherence among women undergoing genetic testing for breast and ovarian cancer is the subject of several ongoing trials, and an area of much needed ongoing study.
References:
- Ropka ME, Wenzel J, Phillips EK, et al.: Uptake rates for breast cancer genetic testing: a systematic review. Cancer Epidemiol Biomarkers Prev 15 (5): 840-55, 2006.
- Schwartz MD, Lerman C, Brogan B, et al.: Utilization of BRCA1/BRCA2 mutation testing in newly diagnosed breast cancer patients. Cancer Epidemiol Biomarkers Prev 14 (4): 1003-7, 2005.
- Kieran S, Loescher LJ, Lim KH: The role of financial factors in acceptance of clinical BRCA genetic testing. Genet Test 11 (1): 101-10, 2007.
- Susswein LR, Skrzynia C, Lange LA, et al.: Increased uptake of BRCA1/2 genetic testing among African American women with a recent diagnosis of breast cancer. J Clin Oncol 26 (1): 32-6, 2008.
- Olaya W, Esquivel P, Wong JH, et al.: Disparities in BRCA testing: when insurance coverage is not a barrier. Am J Surg 198 (4): 562-5, 2009.
- Levy DE, Byfield SD, Comstock CB, et al.: Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and Hispanic women particularly at risk. Genet Med 13 (4): 349-55, 2011.
- Landsbergen K, Verhaak C, Kraaimaat F, et al.: Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer 4 (2): 115-9, 2005.
- Denayer L, Boogaerts A, Philippe K, et al.: BRCA1/2 predictive testing and gender: uptake, motivation and psychological characteristics. Genet Couns 20 (4): 293-305, 2009.
- Meijers-Heijboer EJ, Verhoog LC, Brekelmans CT, et al.: Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet 355 (9220): 2015-20, 2000.
- Wakefield CE, Ratnayake P, Meiser B, et al.: "For all my family's sake, I should go and find out": an Australian report on genetic counseling and testing uptake in individuals at high risk of breast and/or ovarian cancer. Genet Test Mol Biomarkers 15 (6): 379-85, 2011.
- Schlich-Bakker KJ, ten Kroode HF, Wárlám-Rodenhuis CC, et al.: Barriers to participating in genetic counseling and BRCA testing during primary treatment for breast cancer. Genet Med 9 (11): 766-77, 2007.
- Vadaparampil ST, McIntyre J, Quinn GP: Awareness, perceptions, and provider recommendation related to genetic testing for hereditary breast cancer risk among at-risk Hispanic women: similarities and variations by sub-ethnicity. J Genet Couns 19 (6): 618-29, 2010.
- Meiser B: Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psychooncology 14 (12): 1060-74, 2005.
- Pasacreta JV: Psychosocial issues associated with genetic testing for breast and ovarian cancer risk: an integrative review. Cancer Invest 21 (4): 588-623, 2003.
- Simon MS, Petrucelli N: Hereditary breast and ovarian cancer syndrome : the impact of race on uptake of genetic counseling and testing. Methods Mol Biol 471: 487-500, 2009.
- Hann KEJ, Freeman M, Fraser L, et al.: Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC Public Health 17 (1): 503, 2017.
- Meiser B, Gaff C, Julian-Reynier C, et al.: International perspectives on genetic counseling and testing for breast cancer risk. Breast Dis 27: 109-25, 2006-2007.
- Armstrong K, Stopfer J, Calzone K, et al.: What does my doctor think? Preferences for knowing the doctor's opinion among women considering clinical testing for BRCA1/2 mutations. Genet Test 6 (2): 115-8, 2002 Summer.
- McCuaig JM, Greenwood CM, Shuman C, et al.: Breast and ovarian cancer: the forgotten paternal contribution. J Genet Couns 20 (5): 442-9, 2011.
- Burke W, Culver J, Pinsky L, et al.: Genetic assessment of breast cancer risk in primary care practice. Am J Med Genet A 149A (3): 349-56, 2009.
- Mouchawar J, Klein CE, Mullineaux L: Colorado family physicians' knowledge of hereditary breast cancer and related practice. J Cancer Educ 16 (1): 33-7, 2001.
- Yong MC, Zhou XJ, Lee SC: The importance of paternal family history in hereditary breast cancer is underappreciated by health care professionals. Oncology 64 (3): 220-6, 2003.
- Bellcross CA, Leadbetter S, Alford SH, et al.: Prevalence and healthcare actions of women in a large health system with a family history meeting the 2005 USPSTF recommendation for BRCA genetic counseling referral. Cancer Epidemiol Biomarkers Prev 22 (4): 728-35, 2013.
- Weitzel JN, McCaffrey SM, Nedelcu R, et al.: Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis. Arch Surg 138 (12): 1323-8; discussion 1329, 2003.
- Kurian AW, Griffith KA, Hamilton AS, et al.: Genetic Testing and Counseling Among Patients With Newly Diagnosed Breast Cancer. JAMA 317 (5): 531-534, 2017.
- Childers CP, Childers KK, Maggard-Gibbons M, et al.: National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer. J Clin Oncol 35 (34): 3800-3806, 2017.
- Pal T, Bonner D, Kim J, et al.: Early onset breast cancer in a registry-based sample of African-american women: BRCA mutation prevalence, and other personal and system-level clinical characteristics. Breast J 19 (2): 189-92, 2013 Mar-Apr.
- Weldon CB, Trosman JR, Gradishar WJ, et al.: Barriers to the use of personalized medicine in breast cancer. J Oncol Pract 8 (4): e24-31, 2012.
- Hafertepen L, Pastorino A, Morman N, et al.: Barriers to genetic testing in newly diagnosed breast cancer patients: Do surgeons limit testing? Am J Surg 214 (1): 105-110, 2017.
- Katz SJ, Ward KC, Hamilton AS, et al.: Gaps in Receipt of Clinically Indicated Genetic Counseling After Diagnosis of Breast Cancer. J Clin Oncol 36 (12): 1218-1224, 2018.
- Schwartz MD, Peshkin BN, Isaacs C, et al.: Randomized trial of proactive rapid genetic counseling versus usual care for newly diagnosed breast cancer patients. Breast Cancer Res Treat 170 (3): 517-524, 2018.
- Lerman C, Hughes C, Benkendorf JL, et al.: Racial differences in testing motivation and psychological distress following pretest education for BRCA1 gene testing. Cancer Epidemiol Biomarkers Prev 8 (4 Pt 2): 361-7, 1999.
- Armstrong K, Micco E, Carney A, et al.: Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA 293 (14): 1729-36, 2005.
- Kinney AY, Simonsen SE, Baty BJ, et al.: Acceptance of genetic testing for hereditary breast ovarian cancer among study enrollees from an African American kindred. Am J Med Genet A 140 (8): 813-26, 2006.
- Halbert CH, Kessler L, Stopfer JE, et al.: Low rates of acceptance of BRCA1 and BRCA2 test results among African American women at increased risk for hereditary breast-ovarian cancer. Genet Med 8 (9): 576-82, 2006.
- Thompson HS, Valdimarsdottir HB, Duteau-Buck C, et al.: Psychosocial predictors of BRCA counseling and testing decisions among urban African-American women. Cancer Epidemiol Biomarkers Prev 11 (12): 1579-85, 2002.
- Kinney AY, Gammon A, Coxworth J, et al.: Exploring attitudes, beliefs, and communication preferences of Latino community members regarding BRCA1/2 mutation testing and preventive strategies. Genet Med 12 (2): 105-15, 2010.
- Sussner KM, Edwards T, Villagra C, et al.: BRCA genetic counseling among at-risk Latinas in New York City: new beliefs shape new generation. J Genet Couns 24 (1): 134-48, 2015.
- Zimmerman RK, Tabbarah M, Nowalk MP, et al.: Racial differences in beliefs about genetic screening among patients at inner-city neighborhood health centers. J Natl Med Assoc 98 (3): 370-7, 2006.
- MacNew HG, Rudolph R, Brower ST, et al.: Assessing the knowledge and attitudes regarding genetic testing for breast cancer risk in our region of southeastern Georgia. Breast J 16 (2): 189-92, 2010.
- Sussner KM, Thompson HS, Jandorf L, et al.: The influence of acculturation and breast cancer-specific distress on perceived barriers to genetic testing for breast cancer among women of African descent. Psychooncology 18 (9): 945-55, 2009.
- Edwards TA, Thompson HS, Kwate NO, et al.: Association between temporal orientation and attitudes about BRCA1/2 testing among women of African descent with family histories of breast cancer. Patient Educ Couns 72 (2): 276-82, 2008.
- McCarthy AM, Bristol M, Domchek SM, et al.: Health Care Segregation, Physician Recommendation, and Racial Disparities in BRCA1/2 Testing Among Women With Breast Cancer. J Clin Oncol 34 (22): 2610-8, 2016.
- Hurtado-de-Mendoza A, Jackson MC, Anderson L, et al.: The Role of Knowledge on Genetic Counseling and Testing in Black Cancer Survivors at Increased Risk of Carrying a BRCA1/2 Mutation. J Genet Couns 26 (1): 113-121, 2017.
- Cragun D, Weidner A, Lewis C, et al.: Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 123 (13): 2497-2505, 2017.
- Cragun D, Weidner A, Kechik J, et al.: Genetic Testing Across Young Hispanic and Non-Hispanic White Breast Cancer Survivors: Facilitators, Barriers, and Awareness of the Genetic Information Nondiscrimination Act. Genet Test Mol Biomarkers 23 (2): 75-83, 2019.
- Lerman C, Hughes C, Lemon SJ, et al.: What you don't know can hurt you: adverse psychologic effects in members of BRCA1-linked and BRCA2-linked families who decline genetic testing. J Clin Oncol 16 (5): 1650-4, 1998.
- Lodder L, Frets PG, Trijsburg RW, et al.: Attitudes and distress levels in women at risk to carry a BRCA1/BRCA2 gene mutation who decline genetic testing. Am J Med Genet 119A (3): 266-72, 2003.
- Foster C, Evans DG, Eeles R, et al.: Non-uptake of predictive genetic testing for BRCA1/2 among relatives of known carriers: attributes, cancer worry, and barriers to testing in a multicenter clinical cohort. Genet Test 8 (1): 23-9, 2004.
- McInerney-Leo A, Biesecker BB, Hadley DW, et al.: BRCA1/2 testing in hereditary breast and ovarian cancer families II: impact on relationships. Am J Med Genet A 133 (2): 165-9, 2005.
- Patenaude AF: Cancer susceptibility testing: risks, benefits, and personal beliefs. In: Clarke A, ed.: The Genetic Testing of Children. BIOS Scientific, 1998, pp 145-156.
- Richards M: The genetic testing of children: adult attitude's and children's understanding. In: Clarke A, ed.: The Genetic Testing of Children. BIOS Scientific, 1998, pp 169-179.
- Wertz DC, Fanos JH, Reilly PR: Genetic testing for children and adolescents. Who decides? JAMA 272 (11): 875-81, 1994.
- Borry P, Stultiëns L, Nys H, et al.: Attitudes towards predictive genetic testing in minors for familial breast cancer: a systematic review. Crit Rev Oncol Hematol 64 (3): 173-81, 2007.
- Hamann HA, Croyle RT, Venne VL, et al.: Attitudes toward the genetic testing of children among adults in a Utah-based kindred tested for a BRCA1 mutation. Am J Med Genet 92 (1): 25-32, 2000.
- Bradbury AR, Patrick-Miller L, Pawlowski K, et al.: Should genetic testing for BRCA1/2 be permitted for minors? Opinions of BRCA mutation carriers and their adult offspring. Am J Med Genet C Semin Med Genet 148C (1): 70-7, 2008.
- Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. American Society of Human Genetics Board of Directors, American College of Medical Genetics Board of Directors. Am J Hum Genet 57 (5): 1233-41, 1995.
- Michie S, Marteau TM: Predictive genetic testing in children: the need for psychological research. In: Clarke A, ed.: The Genetic Testing of Children. BIOS Scientific, 1998, pp 169-182.
- MacDonald DJ, Lessick M: Hereditary cancers in children and ethical and psychosocial implications. J Pediatr Nurs 15 (4): 217-25, 2000.
- Tercyak KP, Peshkin BN, Streisand R, et al.: Psychological issues among children of hereditary breast cancer gene (BRCA1/2) testing participants. Psychooncology 10 (4): 336-46, 2001 Jul-Aug.
- Winer E, Winer N, Bluman L, et al.: Attitudes and risk perceptions of women with breast cancer considering testing for BRCA1/2. [Abstract] Proceedings of the American Society of Clinical Oncology 16: A1937, 537a, 1997.
- Braithwaite D, Emery J, Walter F, et al.: Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. J Natl Cancer Inst 96 (2): 122-33, 2004.
- Mikkelsen EM, Sunde L, Johansen C, et al.: Psychosocial conditions of women awaiting genetic counseling: a population-based study. J Genet Couns 17 (3): 242-51, 2008.
- Dorval M, Bouchard K, Maunsell E, et al.: Health behaviors and psychological distress in women initiating BRCA1/2 genetic testing: comparison with control population. J Genet Couns 17 (4): 314-26, 2008.
- Wang C, Gonzalez R, Janz N, et al.: The role of cognitive appraisal and worry in BRCA1/2 testing decisions among a clinic population. Psychol Health 22 (6): 719-36, 2007.
- Hallowell N, Statham H, Murton F: Women's understanding of their risk of developing breast/ovarian cancer before and after genetic counseling. J Genet Couns 7 (4): 345-64, 1998.
- MacDonald DJ, Choi J, Ferrell B, et al.: Concerns of women presenting to a comprehensive cancer centre for genetic cancer risk assessment. J Med Genet 39 (7): 526-30, 2002.
- Matloff ET, Moyer A, Shannon KM, et al.: Healthy women with a family history of breast cancer: impact of a tailored genetic counseling intervention on risk perception, knowledge, and menopausal therapy decision making. J Womens Health (Larchmt) 15 (7): 843-56, 2006.
- Meiser B, Price MA, Butow PN, et al.: Misperceptions of ovarian cancer risk in women at increased risk for hereditary ovarian cancer. Fam Cancer 13 (2): 153-62, 2014.
- Bluman LG, Rimer BK, Berry DA, et al.: Attitudes, knowledge, and risk perceptions of women with breast and/or ovarian cancer considering testing for BRCA1 and BRCA2. J Clin Oncol 17 (3): 1040-6, 1999.
- Iglehart JD, Miron A, Rimer BK, et al.: Overestimation of hereditary breast cancer risk. Ann Surg 228 (3): 375-84, 1998.
- Bluman LG, Rimer BK, Regan Sterba K, et al.: Attitudes, knowledge, risk perceptions and decision-making among women with breast and/or ovarian cancer considering testing for BRCA1 and BRCA2 and their spouses. Psychooncology 12 (5): 410-27, 2003 Jul-Aug.
- McCaul KD, O'Donnell SM: Naive beliefs about breast cancer risk. Womens Health 4 (1): 93-101, 1998 Spring.
- Huiart L, Eisinger F, Stoppa-Lyonnet D, et al.: Effects of genetic consultation on perception of a family risk of breast/ovarian cancer and determinants of inaccurate perception after the consultation. J Clin Epidemiol 55 (7): 665-75, 2002.
- Davis S, Stewart S, Bloom J: Increasing the accuracy of perceived breast cancer risk: results from a randomized trial with Cancer Information Service callers. Prev Med 39 (1): 64-73, 2004.
- Katapodi MC, Dodd MJ, Lee KA, et al.: Underestimation of breast cancer risk: influence on screening behavior. Oncol Nurs Forum 36 (3): 306-14, 2009.
- Lindberg NM, Wellisch D: Anxiety and compliance among women at high risk for breast cancer. Ann Behav Med 23 (4): 298-303, 2001 Fall.
- Ritvo P, Irvine J, Robinson G, et al.: Psychological adjustment to familial-genetic risk assessment for ovarian cancer: predictors of nonadherence to surveillance recommendations. Gynecol Oncol 84 (1): 72-80, 2002.
- Meiser B, Halliday JL: What is the impact of genetic counselling in women at increased risk of developing hereditary breast cancer? A meta-analytic review. Soc Sci Med 54 (10): 1463-70, 2002.
- Green J, Richards M, Murton F, et al.: Family communication and genetic counseling: the case of hereditary breast and ovarian cancer. J Genet Couns 6 (1): 45-60, 1997.
- Quillin JM, Ramakrishnan V, Borzelleca J, et al.: Paternal relatives and family history of breast cancer. Am J Prev Med 31 (3): 265-8, 2006.
- O'Neill SM, Rubinstein WS, Wang C, et al.: Familial risk for common diseases in primary care: the Family Healthware Impact Trial. Am J Prev Med 36 (6): 506-14, 2009.
- Rubinstein WS, O'neill SM, Rothrock N, et al.: Components of family history associated with women's disease perceptions for cancer: a report from the Family Healthware™ Impact Trial. Genet Med 13 (1): 52-62, 2011.
- Theis B, Boyd N, Lockwood G, et al.: Accuracy of family cancer history in breast cancer patients. Eur J Cancer Prev 3 (4): 321-7, 1994.
- Breuer B, Kash KM, Rosenthal G, et al.: Reporting bilaterality status in first-degree relatives with breast cancer: a validity study. Genet Epidemiol 10 (4): 245-56, 1993.
- Parent ME, Ghadirian P, Lacroix A, et al.: The reliability of recollections of family history: implications for the medical provider. J Cancer Educ 12 (2): 114-20, 1997 Summer.
- Kelly KM, Shedlosky-Shoemaker R, Porter K, et al.: Cancer family history reporting: impact of method and psychosocial factors. J Genet Couns 16 (3): 373-82, 2007.
- Kerber RA, Slattery ML: Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol 146 (3): 244-8, 1997.
- Kerr B, Foulkes WD, Cade D, et al.: False family history of breast cancer in the family cancer clinic. Eur J Surg Oncol 24 (4): 275-9, 1998.
- Schwartz MD, Peshkin BN, Hughes C, et al.: Impact of BRCA1/BRCA2 mutation testing on psychologic distress in a clinic-based sample. J Clin Oncol 20 (2): 514-20, 2002.
- Mancini J, Noguès C, Adenis C, et al.: Impact of an information booklet on satisfaction and decision-making about BRCA genetic testing. Eur J Cancer 42 (7): 871-81, 2006.
- Green MJ, Biesecker BB, McInerney AM, et al.: An interactive computer program can effectively educate patients about genetic testing for breast cancer susceptibility. Am J Med Genet 103 (1): 16-23, 2001.
- Green MJ, Peterson SK, Baker MW, et al.: Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. JAMA 292 (4): 442-52, 2004.
- Green MJ, McInerney AM, Biesecker BB, et al.: Education about genetic testing for breast cancer susceptibility: patient preferences for a computer program or genetic counselor. Am J Med Genet 103 (1): 24-31, 2001.
- Dabney MK, Huelsman K: Counseling by computer: breast cancer risk and genetic testing. Developed by the University of Wisconsin-Madison Department of Medicine and the Program in Medical Ethics. Genet Test 4 (1): 43-4, 2000.
- Green MJ, Peterson SK, Baker MW, et al.: Use of an educational computer program before genetic counseling for breast cancer susceptibility: effects on duration and content of counseling sessions. Genet Med 7 (4): 221-9, 2005.
- Wang C, Gonzalez R, Milliron KJ, et al.: Genetic counseling for BRCA1/2: a randomized controlled trial of two strategies to facilitate the education and counseling process. Am J Med Genet A 134 (1): 66-73, 2005.
- Joseph G, Beattie MS, Lee R, et al.: Pre-counseling education for low literacy women at risk of Hereditary Breast and Ovarian Cancer (HBOC): patient experiences using the Cancer Risk Education Intervention Tool (CREdIT). J Genet Couns 19 (5): 447-62, 2010.
- Albada A, van Dulmen S, Lindhout D, et al.: A pre-visit tailored website enhances counselees' realistic expectations and knowledge and fulfils information needs for breast cancer genetic counselling. Fam Cancer 11 (1): 85-95, 2012.
- Baty BJ, Kinney AY, Ellis SM: Developing culturally sensitive cancer genetics communication aids for African Americans. Am J Med Genet 118A (2): 146-55, 2003.
- Permuth-Wey J, Vadaparampil S, Rumphs A, et al.: Development of a culturally tailored genetic counseling booklet about hereditary breast and ovarian cancer for Black women. Am J Med Genet A 152A (4): 836-45, 2010.
- Pal T, Stowe C, Cole A, et al.: Evaluation of phone-based genetic counselling in African American women using culturally tailored visual aids. Clin Genet 78 (2): 124-31, 2010.
- Calzone KA: Predisposition testing for breast and ovarian cancer susceptibility. Semin Oncol Nurs 13 (2): 82-90, 1997.
- Smith KR, West JA, Croyle RT, et al.: Familial context of genetic testing for cancer susceptibility: moderating effect of siblings' test results on psychological distress one to two weeks after BRCA1 mutation testing. Cancer Epidemiol Biomarkers Prev 8 (4 Pt 2): 385-92, 1999.
- Wylie JE, Smith KR, Botkin JR: Effects of spouses on distress experienced by BRCA1 mutation carriers over time. Am J Med Genet 119C (1): 35-44, 2003.
- Kash KM, Holland JC, Halper MS, et al.: Psychological distress and surveillance behaviors of women with a family history of breast cancer. J Natl Cancer Inst 84 (1): 24-30, 1992.
- Lerman C, Schwartz M: Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Res Treat 28 (2): 145-55, 1993.
- Kelly PT: Understanding Breast Cancer Risk. Temple University Press, 1991.
- Baty BJ, Venne VL, McDonald J, et al.: BRCA1 testing: genetic counseling protocol development and counseling issues. J Genet Couns 6 (2): 223-44, 1997.
- Richards MP, Hallowell N, Green JM, et al.: Counseling families with hereditary breast and ovarian cancer: a psychosocial perspective. J Genet Couns 4 (3): 219-33, 1995.
- Hoskins KF, Stopfer JE, Calzone KA, et al.: Assessment and counseling for women with a family history of breast cancer. A guide for clinicians. JAMA 273 (7): 577-85, 1995.
- Schneider KA: Genetic counseling for BRCA1/BRCA2 testing. Genet Test 1 (2): 91-8, 1997.
- McKinnon WC, Baty BJ, Bennett RL, et al.: Predisposition genetic testing for late-onset disorders in adults. A position paper of the National Society of Genetic Counselors. JAMA 278 (15): 1217-20, 1997.
- Cummings S, Olopade O: Predisposition testing for inherited breast cancer. Oncology (Huntingt) 12 (8): 1227-41; discussion 1241-2, 1998.
- Lipkus IM, Klein WM, Rimer BK: Communicating breast cancer risks to women using different formats. Cancer Epidemiol Biomarkers Prev 10 (8): 895-8, 2001.
- Butow PN, Lobb EA: Analyzing the process and content of genetic counseling in familial breast cancer consultations. J Genet Couns 13 (5): 403-24, 2004.
- Lerman C, Audrain J, Croyle RT: DNA-testing for heritable breast cancer risks: lessons from traditional genetic counseling. Ann Behav Med 16(4): 327-333, 1994.
- Pieterse AH, van Dulmen AM, Beemer FA, et al.: Cancer genetic counseling: communication and counselees' post-visit satisfaction, cognitions, anxiety, and needs fulfillment. J Genet Couns 16 (1): 85-96, 2007.
- Hamilton JG, Lobel M, Moyer A: Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta-analytic review. Health Psychol 28 (4): 510-8, 2009.
- Beran TM, Stanton AL, Kwan L, et al.: The trajectory of psychological impact in BRCA1/2 genetic testing: does time heal? Ann Behav Med 36 (2): 107-16, 2008.
- Bosch N, Junyent N, Gadea N, et al.: What factors may influence psychological well being at three months and one year post BRCA genetic result disclosure? Breast 21 (6): 755-60, 2012.
- O'Neill SC, Rini C, Goldsmith RE, et al.: Distress among women receiving uninformative BRCA1/2 results: 12-month outcomes. Psychooncology 18 (10): 1088-96, 2009.
- Oberguggenberger A, Sztankay M, Morscher RJ, et al.: Psychosocial outcomes and counselee satisfaction following genetic counseling for hereditary breast and ovarian cancer: A patient-reported outcome study. J Psychosom Res 89: 39-45, 2016.
- Foster C, Watson M, Eeles R, et al.: Predictive genetic testing for BRCA1/2 in a UK clinical cohort: three-year follow-up. Br J Cancer 96 (5): 718-24, 2007.
- Halbert CH, Stopfer JE, McDonald J, et al.: Long-term reactions to genetic testing for BRCA1 and BRCA2 mutations: does time heal women's concerns? J Clin Oncol 29 (32): 4302-6, 2011.
- Graves KD, Vegella P, Poggi EA, et al.: Long-term psychosocial outcomes of BRCA1/BRCA2 testing: differences across affected status and risk-reducing surgery choice. Cancer Epidemiol Biomarkers Prev 21 (3): 445-55, 2012.
- Cella D, Hughes C, Peterman A, et al.: A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol 21 (6): 564-72, 2002.
- Shkedi-Rafid S, Gabai-Kapara E, Grinshpun-Cohen J, et al.: BRCA genetic testing of individuals from families with low prevalence of cancer: experiences of carriers and implications for population screening. Genet Med 14 (7): 688-94, 2012.
- Metcalfe KA, Poll A, Llacuachaqui M, et al.: Patient satisfaction and cancer-related distress among unselected Jewish women undergoing genetic testing for BRCA1 and BRCA2. Clin Genet 78 (5): 411-7, 2010.
- Metcalfe KA, Mian N, Enmore M, et al.: Long-term follow-up of Jewish women with a BRCA1 and BRCA2 mutation who underwent population genetic screening. Breast Cancer Res Treat 133 (2): 735-40, 2012.
- Armstrong J, Toscano M, Kotchko N, et al.: Utilization and Outcomes of BRCA Genetic Testing and Counseling in a National Commercially Insured Population: The ABOUT Study. JAMA Oncol 1 (9): 1251-60, 2015.
- Dohany L, Gustafson S, Ducaine W, et al.: Psychological distress with direct-to-consumer genetic testing: a case report of an unexpected BRCA positive test result. J Genet Couns 21 (3): 399-401, 2012.
- Mahon SM: Impact of direct-to-consumer genetic testing. J Oncol Pract 8 (4): 260, 2012.
- Jeffers L, Morrison PJ, McCaughan E, et al.: Maximising survival: the main concern of women with hereditary breast and ovarian cancer who undergo genetic testing for BRCA1/2. Eur J Oncol Nurs 18 (4): 411-8, 2014.
- Francke U, Dijamco C, Kiefer AK, et al.: Dealing with the unexpected: consumer responses to direct-access BRCA mutation testing. PeerJ 1: e8, 2013.
- Meiser B, Quinn VF, Gleeson M, et al.: When knowledge of a heritable gene mutation comes out of the blue: treatment-focused genetic testing in women newly diagnosed with breast cancer. Eur J Hum Genet 24 (11): 1517-1523, 2016.
- Ardern-Jones A, Kenen R, Eeles R: Too much, too soon? Patients and health professionals' views concerning the impact of genetic testing at the time of breast cancer diagnosis in women under the age of 40. Eur J Cancer Care (Engl) 14 (3): 272-81, 2005.
- Wevers MR, Hahn DE, Verhoef S, et al.: Breast cancer genetic counseling after diagnosis but before treatment: a pilot study on treatment consequences and psychological impact. Patient Educ Couns 89 (1): 89-95, 2012.
- Zilliacus E, Meiser B, Gleeson M, et al.: Are we being overly cautious? A qualitative inquiry into the experiences and perceptions of treatment-focused germline BRCA genetic testing amongst women recently diagnosed with breast cancer. Support Care Cancer 20 (11): 2949-58, 2012.
- Tercyak KP, Peshkin BN, Brogan BM, et al.: Quality of life after contralateral prophylactic mastectomy in newly diagnosed high-risk breast cancer patients who underwent BRCA1/2 gene testing. J Clin Oncol 25 (3): 285-91, 2007.
- Metcalfe KA, Eisen A, Wright F, et al.: Impact of rapid genetic testing for BRCA1 and BRCA2 at time of breast cancer diagnosis on psychosocial functioning. Breast Cancer Res Treat 191 (3): 631-641, 2022.
- Wevers MR, Ausems MG, Verhoef S, et al.: Does rapid genetic counseling and testing in newly diagnosed breast cancer patients cause additional psychosocial distress? results from a randomized clinical trial. Genet Med 18 (2): 137-44, 2016.
- Wevers MR, Aaronson NK, Bleiker EMA, et al.: Rapid genetic counseling and testing in newly diagnosed breast cancer: Patients' and health professionals' attitudes, experiences, and evaluation of effects on treatment decision making. J Surg Oncol 116 (8): 1029-1039, 2017.
- Conley CC, Ketcher D, Reblin M, et al.: The big reveal: Family disclosure patterns of BRCA genetic test results among young Black women with invasive breast cancer. J Genet Couns 29 (3): 410-422, 2020.
- Patenaude AF, Dorval M, DiGianni LS, et al.: Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol 24 (4): 700-6, 2006.
- Lerman C, Peshkin BN, Hughes C, et al.: Family disclosure in genetic testing for cancer susceptibility: determinants and consequences. Journal of Health Care Law and Policy 1 (2): 353-73, 1998.
- Elrick A, Ashida S, Ivanovich J, et al.: Psychosocial and Clinical Factors Associated with Family Communication of Cancer Genetic Test Results among Women Diagnosed with Breast Cancer at a Young Age. J Genet Couns 26 (1): 173-181, 2017.
- Finlay E, Stopfer JE, Burlingame E, et al.: Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test 12 (1): 81-91, 2008.
- Hughes C, Lerman C, Schwartz M, et al.: All in the family: evaluation of the process and content of sisters' communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet 107 (2): 143-50, 2002.
- Baars JE, Ausems MG, van Riel E, et al.: Communication Between Breast Cancer Patients Who Received Inconclusive Genetic Test Results and Their Daughters and Sisters Years After Testing. J Genet Couns 25 (3): 461-71, 2016.
- Wagner Costalas J, Itzen M, Malick J, et al.: Communication of BRCA1 and BRCA2 results to at-risk relatives: a cancer risk assessment program's experience. Am J Med Genet 119C (1): 11-8, 2003.
- MacDonald DJ, Sarna L, van Servellen G, et al.: Selection of family members for communication of cancer risk and barriers to this communication before and after genetic cancer risk assessment. Genet Med 9 (5): 275-82, 2007.
- Kenen R, Arden-Jones A, Eeles R: Healthy women from suspected hereditary breast and ovarian cancer families: the significant others in their lives. Eur J Cancer Care (Engl) 13 (2): 169-79, 2004.
- Claes E, Evers-Kiebooms G, Boogaerts A, et al.: Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet 116A (1): 11-9, 2003.
- Foster C, Eeles R, Ardern-Jones A, et al.: Juggling roles and expectations: dilemmas faced by women talking to relatives about cancer and genetic testing. Psychol Health 19 (4): 439-55, 2004.
- Kenen R, Arden-Jones A, Eeles R: We are talking, but are they listening? Communication patterns in families with a history of breast/ovarian cancer (HBOC). Psychooncology 13 (5): 335-45, 2004.
- Segal J, Esplen MJ, Toner B, et al.: An investigation of the disclosure process and support needs of BRCA1 and BRCA2 carriers. Am J Med Genet A 125 (3): 267-72, 2004.
- Bradbury AR, Dignam JJ, Ibe CN, et al.: How often do BRCA mutation carriers tell their young children of the family's risk for cancer? A study of parental disclosure of BRCA mutations to minors and young adults. J Clin Oncol 25 (24): 3705-11, 2007.
- Bradbury AR, Patrick-Miller L, Pawlowski K, et al.: Learning of your parent's BRCA mutation during adolescence or early adulthood: a study of offspring experiences. Psychooncology 18 (2): 200-8, 2009.
- Manne S, Audrain J, Schwartz M, et al.: Associations between relationship support and psychological reactions of participants and partners to BRCA1 and BRCA2 testing in a clinic-based sample. Ann Behav Med 28 (3): 211-25, 2004.
- Daly MB, Montgomery S, Bingler R, et al.: Communicating genetic test results within the family: Is it lost in translation? A survey of relatives in the randomized six-step study. Fam Cancer 15 (4): 697-706, 2016.
- McAllister MF, Evans DG, Ormiston W, et al.: Men in breast cancer families: a preliminary qualitative study of awareness and experience. J Med Genet 35 (9): 739-44, 1998.
- Liede A, Metcalfe K, Hanna D, et al.: Evaluation of the needs of male carriers of mutations in BRCA1 or BRCA2 who have undergone genetic counseling. Am J Hum Genet 67 (6): 1494-504, 2000.
- Metcalfe KA, Liede A, Trinkaus M, et al.: Evaluation of the needs of spouses of female carriers of mutations in BRCA1 and BRCA2. Clin Genet 62 (6): 464-9, 2002.
- Mireskandari S, Sherman KA, Meiser B, et al.: Psychological adjustment among partners of women at high risk of developing breast/ovarian cancer. Genet Med 9 (5): 311-20, 2007.
- Sherman KA, Kasparian NA, Mireskandari S: Psychological adjustment among male partners in response to women's breast/ovarian cancer risk: a theoretical review of the literature. Psychooncology 19 (1): 1-11, 2010.
- Daly MB: The impact of social roles on the experience of men in BRCA1/2 families: implications for counseling. J Genet Couns 18 (1): 42-8, 2009.
- DudokdeWit AC, Tibben A, Frets PG, et al.: Males at-risk for the BRCA1 gene, the psychological impact. Psychooncology 5(3): 251-257, 1996.
- Lodder L, Frets PG, Trijsburg RW, et al.: Men at risk of being a mutation carrier for hereditary breast/ovarian cancer: an exploration of attitudes and psychological functioning during genetic testing. Eur J Hum Genet 9 (7): 492-500, 2001.
- Lerman C, Hughes C, Croyle RT, et al.: Prophylactic surgery decisions and surveillance practices one year following BRCA1/2 testing. Prev Med 31 (1): 75-80, 2000.
- Hughes C, Lynch H, Durham C, et al.: Communication of BRCA1/2 Test Results in Hereditary Breast Cancer Families. Cancer Research in Therapy and Control Vol. 8, 1999, pp. 51-59.
- Tercyak KP, Hughes C, Main D, et al.: Parental communication of BRCA1/2 genetic test results to children. Patient Educ Couns 42 (3): 213-24, 2001.
- Tercyak KP, Peshkin BN, DeMarco TA, et al.: Parent-child factors and their effect on communicating BRCA1/2 test results to children. Patient Educ Couns 47 (2): 145-53, 2002.
- McGivern B, Everett J, Yager GG, et al.: Family communication about positive BRCA1 and BRCA2 genetic test results. Genet Med 6 (6): 503-9, 2004 Nov-Dec.
- Bradbury AR, Patrick-Miller L, Schwartz L, et al.: Psychosocial Adjustment in School-age Girls With a Family History of Breast Cancer. Pediatrics 136 (5): 927-37, 2015.
- Bradbury AR, Patrick-Miller L, Schwartz LA, et al.: Psychosocial Adjustment and Perceived Risk Among Adolescent Girls From Families With BRCA1/2 or Breast Cancer History. J Clin Oncol 34 (28): 3409-16, 2016.
- Tercyak KP, Peshkin BN, Demarco TA, et al.: Information needs of mothers regarding communicating BRCA1/2 cancer genetic test results to their children. Genet Test 11 (3): 249-55, 2007.
- Wertz DC: International perspectives. In: Clarke A, ed.: The Genetic Testing of Children. BIOS Scientific, 1998, pp 271-287.
- Benkendorf JL, Reutenauer JE, Hughes CA, et al.: Patients' attitudes about autonomy and confidentiality in genetic testing for breast-ovarian cancer susceptibility. Am J Med Genet 73 (3): 296-303, 1997.
- Staton AD, Kurian AW, Cobb K, et al.: Cancer risk reduction and reproductive concerns in female BRCA1/2 mutation carriers. Fam Cancer 7 (2): 179-86, 2008.
- Friedman LC, Kramer RM: Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr (34): 83-6, 2005.
- Smith KR, Ellington L, Chan AY, et al.: Fertility intentions following testing for a BRCA1 gene mutation. Cancer Epidemiol Biomarkers Prev 13 (5): 733-40, 2004.
- Quinn G, Vadaparampil S, Wilson C, et al.: Attitudes of high-risk women toward preimplantation genetic diagnosis. Fertil Steril 91 (6): 2361-8, 2009.
- Cunniff C; American Academy of Pediatrics Committee on Genetics: Prenatal screening and diagnosis for pediatricians. Pediatrics 114 (3): 889-94, 2004.
- Rappaport VJ: Prenatal diagnosis and genetic screening--integration into prenatal care. Obstet Gynecol Clin North Am 35 (3): 435-58, ix, 2008.
- Baruch S, Kaufman D, Hudson KL: Genetic testing of embryos: practices and perspectives of US in vitro fertilization clinics. Fertil Steril 89 (5): 1053-8, 2008.
- Ogilvie CM, Braude PR, Scriven PN: Preimplantation genetic diagnosis--an overview. J Histochem Cytochem 53 (3): 255-60, 2005.
- Vadaparampil ST, Quinn GP, Knapp C, et al.: Factors associated with preimplantation genetic diagnosis acceptance among women concerned about hereditary breast and ovarian cancer. Genet Med 11 (10): 757-65, 2009.
- Quinn GP, Vadaparampil ST, King LM, et al.: Conflict between values and technology: perceptions of preimplantation genetic diagnosis among women at increased risk for hereditary breast and ovarian cancer. Fam Cancer 8 (4): 441-9, 2009.
- Chan JL, Johnson LNC, Sammel MD, et al.: Reproductive Decision-Making in Women with BRCA1/2 Mutations. J Genet Couns 26 (3): 594-603, 2017.
- Menon U, Harper J, Sharma A, et al.: Views of BRCA gene mutation carriers on preimplantation genetic diagnosis as a reproductive option for hereditary breast and ovarian cancer. Hum Reprod 22 (6): 1573-7, 2007.
- Fortuny D, Balmaña J, Graña B, et al.: Opinion about reproductive decision making among individuals undergoing BRCA1/2 genetic testing in a multicentre Spanish cohort. Hum Reprod 24 (4): 1000-6, 2009.
- Julian-Reynier C, Fabre R, Coupier I, et al.: BRCA1/2 carriers: their childbearing plans and theoretical intentions about having preimplantation genetic diagnosis and prenatal diagnosis. Genet Med 14 (5): 527-34, 2012.
- Ormondroyd E, Donnelly L, Moynihan C, et al.: Attitudes to reproductive genetic testing in women who had a positive BRCA test before having children: a qualitative analysis. Eur J Hum Genet 20 (1): 4-10, 2012.
- Quinn GP, Vadaparampil ST, Miree CA, et al.: High risk men's perceptions of pre-implantation genetic diagnosis for hereditary breast and ovarian cancer. Hum Reprod 25 (10): 2543-50, 2010.
- Struewing JP, Abeliovich D, Peretz T, et al.: The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet 11 (2): 198-200, 1995.
- Rothenberg KH: Breast cancer, the genetic "quick fix," and the Jewish community. Ethical, legal, and social challenges. Health Matrix Clevel 7 (1): 97-124, 1997 Winter.
- Foster MW, Bernsten D, Carter TH: A model agreement for genetic research in socially identifiable populations. Am J Hum Genet 63 (3): 696-702, 1998.
- Burhansstipanov L, Bemis LT, Dignan MB: Native American cancer education: genetic and cultural issues. J Cancer Educ 16 (3): 142-5, 2001 Autumn.
- Hughes C, Fasaye GA, LaSalle VH, et al.: Sociocultural influences on participation in genetic risk assessment and testing among African American women. Patient Educ Couns 51 (2): 107-14, 2003.
- Julian-Reynier CM, Bouchard LJ, Evans DG, et al.: Women's attitudes toward preventive strategies for hereditary breast or ovarian carcinoma differ from one country to another: differences among English, French, and Canadian women. Cancer 92 (4): 959-68, 2001.
- Phillips KA, Warner E, Meschino WS, et al.: Perceptions of Ashkenazi Jewish breast cancer patients on genetic testing for mutations in BRCA1 and BRCA2. Clin Genet 57 (5): 376-83, 2000.
- Vadaparampil ST, Quinn GP, Small BJ, et al.: A pilot study of hereditary breast and ovarian knowledge among a multiethnic group of Hispanic women with a personal or family history of cancer. Genet Test Mol Biomarkers 14 (1): 99-106, 2010.
- Halbert CH, Kessler L, Troxel AB, et al.: Effect of genetic counseling and testing for BRCA1 and BRCA2 mutations in African American women: a randomized trial. Public Health Genomics 13 (7-8): 440-8, 2010.
- Freedman TG: Genetic susceptibility testing: ethical and social quandaries. Health Soc Work 23 (3): 214-22, 1998.
- Hubbard R, Lewontin RC: Pitfalls of genetic testing. N Engl J Med 334 (18): 1192-4, 1996.
- Parens E: Glad and terrified: on the ethics of BRACA1 and 2 testing. Cancer Invest 14 (4): 405-11, 1996.
- Winter PR, Wiesner GL, Finnegan J, et al.: Notification of a family history of breast cancer: issues of privacy and confidentiality. Am J Med Genet 66 (1): 1-6, 1996.
- Geller G, Doksum T, Bernhardt BA, et al.: Participation in breast cancer susceptibility testing protocols: influence of recruitment source, altruism, and family involvement on women's decisions. Cancer Epidemiol Biomarkers Prev 8 (4 Pt 2): 377-83, 1999.
- Rimer BK, Schildkraut JM, Lerman C, et al.: Participation in a women's breast cancer risk counseling trial. Who participates? Who declines? High Risk Breast Cancer Consortium. Cancer 77 (11): 2348-55, 1996.
- Robson ME, Storm CD, Weitzel J, et al.: American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol 28 (5): 893-901, 2010.
- Burke W, Daly M, Garber J, et al.: Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA 277 (12): 997-1003, 1997.
- Durfy SJ, Buchanan TE, Burke W: Testing for inherited susceptibility to breast cancer: a survey of informed consent forms for BRCA1 and BRCA2 mutation testing. Am J Med Genet 75 (1): 82-7, 1998.
- Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility, Adopted on February 20, 1996. J Clin Oncol 14 (5): 1730-6; discussion 1737-40, 1996.
- Hallowell N, Foster C, Eeles R, et al.: Balancing autonomy and responsibility: the ethics of generating and disclosing genetic information. J Med Ethics 29 (2): 74-9; discussion 80-3, 2003.
- Krassuski L, Vennedey V, Stock S, et al.: Effectiveness of decision aids for female BRCA1 and BRCA2 mutation carriers: a systematic review. BMC Med Inform Decis Mak 19 (1): 154, 2019.
- Metcalfe KA, Pal T, Narod SA, et al.: Theory-based behavior change intervention to increase uptake of risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 pathogenic variant: The PREVENT randomized controlled trial. Cancer Med 12 (17): 18246-18257, 2023.
- Metcalfe K, Eisen A, Senter L, et al.: International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br J Cancer 121 (1): 15-21, 2019.
- Marcinkute R, Woodward ER, Gandhi A, et al.: Uptake and efficacy of bilateral risk reducing surgery in unaffected female BRCA1 and BRCA2 carriers. J Med Genet 59 (2): 133-140, 2022.
- Schwartz MD, Isaacs C, Graves KD, et al.: Long-term outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer 118 (2): 510-7, 2012.
- Garcia C, Wendt J, Lyon L, et al.: Risk management options elected by women after testing positive for a BRCA mutation. Gynecol Oncol 132 (2): 428-33, 2014.
- Schwartz MD, Lerman C, Brogan B, et al.: Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol 22 (10): 1823-9, 2004.
- Armstrong J, Lynch K, Virgo KS, et al.: Utilization, Timing, and Outcomes of BRCA Genetic Testing Among Women With Newly Diagnosed Breast Cancer From a National Commercially Insured Population: The ABOARD Study. JCO Oncol Pract 17 (2): e226-e235, 2021.
- Bradbury AR, Ibe CN, Dignam JJ, et al.: Uptake and timing of bilateral prophylactic salpingo-oophorectomy among BRCA1 and BRCA2 mutation carriers. Genet Med 10 (3): 161-166, 2008.
- Park SY, Kim Y, Kim S: Factors associated with the decision to undergo risk-reducing salpingo-oophorectomy among women at high risk for hereditary breast and ovarian cancer: a systematic review. Korean J Women Health Nurs 26 (4): 285-299, 2020.
- Cortesi L, Razzaboni E, Toss A, et al.: A rapid genetic counselling and testing in newly diagnosed breast cancer is associated with high rate of risk-reducing mastectomy in BRCA1/2-positive Italian women. Ann Oncol 25 (1): 57-63, 2014.
- Rosenberg SM, Ruddy KJ, Tamimi RM, et al.: BRCA1 and BRCA2 Mutation Testing in Young Women With Breast Cancer. JAMA Oncol 2 (6): 730-6, 2016.
- Howard-McNatt M, Schroll RW, Hurt GJ, et al.: Contralateral prophylactic mastectomy in breast cancer patients who test negative for BRCA mutations. Am J Surg 202 (3): 298-302, 2011.
- Perez L, Webster E, Bull L, et al.: Patient perspectives on risk-reducing salpingectomy with delayed oophorectomy for ovarian cancer risk-reduction: A systematic review of the literature. Gynecol Oncol 173: 106-113, 2023.
- Nebgen DR, Hurteau J, Holman LL, et al.: Bilateral salpingectomy with delayed oophorectomy for ovarian cancer risk reduction: A pilot study in women with BRCA1/2 mutations. Gynecol Oncol 150 (1): 79-84, 2018.
- MacDonald DJ, Sarna L, Uman GC, et al.: Cancer screening and risk-reducing behaviors of women seeking genetic cancer risk assessment for breast and ovarian cancers. Oncol Nurs Forum 33 (2): E27-35, 2006.
- Litton JK, Westin SN, Ready K, et al.: Perception of screening and risk reduction surgeries in patients tested for a BRCA deleterious mutation. Cancer 115 (8): 1598-604, 2009.
- Tyndel S, Austoker J, Henderson BJ, et al.: What is the psychological impact of mammographic screening on younger women with a family history of breast cancer? Findings from a prospective cohort study by the PIMMS Management Group. J Clin Oncol 25 (25): 3823-30, 2007.
- Rees G, Young MA, Gaff C, et al.: A qualitative study of health professionals' views regarding provision of information about health-protective behaviors during genetic consultation for breast cancer. J Genet Couns 15 (2): 95-104, 2006.
- Lodder LN, Frets PG, Trijsburg RW, et al.: One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res Treat 73 (2): 97-112, 2002.
- van Oostrom I, Meijers-Heijboer H, Lodder LN, et al.: Long-term psychological impact of carrying a BRCA1/2 mutation and prophylactic surgery: a 5-year follow-up study. J Clin Oncol 21 (20): 3867-74, 2003.
- Bresser PJ, Seynaeve C, Van Gool AR, et al.: The course of distress in women at increased risk of breast and ovarian cancer due to an (identified) genetic susceptibility who opt for prophylactic mastectomy and/or salpingo-oophorectomy. Eur J Cancer 43 (1): 95-103, 2007.
- Frost MH, Schaid DJ, Sellers TA, et al.: Long-term satisfaction and psychological and social function following bilateral prophylactic mastectomy. JAMA 284 (3): 319-24, 2000.
- Metcalfe KA, Esplen MJ, Goel V, et al.: Psychosocial functioning in women who have undergone bilateral prophylactic mastectomy. Psychooncology 13 (1): 14-25, 2004.
- Schlich-Bakker KJ, Ausems MG, Schipper M, et al.: BRCA1/2 mutation testing in breast cancer patients: a prospective study of the long-term psychological impact of approach during adjuvant radiotherapy. Breast Cancer Res Treat 109 (3): 507-14, 2008.
- Frost MH, Slezak JM, Tran NV, et al.: Satisfaction after contralateral prophylactic mastectomy: the significance of mastectomy type, reconstructive complications, and body appearance. J Clin Oncol 23 (31): 7849-56, 2005.
- Schwartz MD: Contralateral prophylactic mastectomy: efficacy, satisfaction, and regret. J Clin Oncol 23 (31): 7777-9, 2005.
- Metcalfe KA, Cil TD, Semple JL, et al.: Long-Term Psychosocial Functioning in Women with Bilateral Prophylactic Mastectomy: Does Preservation of the Nipple-Areolar Complex Make a Difference? Ann Surg Oncol 22 (10): 3324-30, 2015.
- Yao K, Liederbach E, Tang R, et al.: Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol 22 (2): 370-6, 2015.
- Geiger AM, Nekhlyudov L, Herrinton LJ, et al.: Quality of life after bilateral prophylactic mastectomy. Ann Surg Oncol 14 (2): 686-94, 2007.
- Isern AE, Tengrup I, Loman N, et al.: Aesthetic outcome, patient satisfaction, and health-related quality of life in women at high risk undergoing prophylactic mastectomy and immediate breast reconstruction. J Plast Reconstr Aesthet Surg 61 (10): 1177-87, 2008.
- Kenen RH, Shapiro PJ, Hantsoo L, et al.: Women with BRCA1 or BRCA2 mutations renegotiating a post-prophylactic mastectomy identity: self-image and self-disclosure. J Genet Couns 16 (6): 789-98, 2007.
- Altschuler A, Nekhlyudov L, Rolnick SJ, et al.: Positive, negative, and disparate--women's differing long-term psychosocial experiences of bilateral or contralateral prophylactic mastectomy. Breast J 14 (1): 25-32, 2008 Jan-Feb.
- Patenaude AF, Orozco S, Li X, et al.: Support needs and acceptability of psychological and peer consultation: attitudes of 108 women who had undergone or were considering prophylactic mastectomy. Psychooncology 17 (8): 831-43, 2008.
- Hall E, Finch A, Jacobson M, et al.: Effects of bilateral salpingo-oophorectomy on menopausal symptoms and sexual functioning among women with a BRCA1 or BRCA2 mutation. Gynecol Oncol 152 (1): 145-150, 2019.
- Powell CB, Alabaster A, Le A, et al.: Sexual function, menopausal symptoms, depression and cancer worry in women with BRCA mutations. Psychooncology 29 (2): 331-338, 2020.
- Terra L, Lee Meeuw Kjoe PR, Agelink van Rentergem JA, et al.: Long-term effects of premenopausal risk-reducing salpingo-oophorectomy on cognition in women with high familial risk of ovarian cancer: A cross-sectional study. BJOG 130 (8): 968-977, 2023.
- Isaacs C, Peshkin BN, Schwartz M, et al.: Breast and ovarian cancer screening practices in healthy women with a strong family history of breast or ovarian cancer. Breast Cancer Res Treat 71 (2): 103-12, 2002.
- Peshkin BN, Schwartz MD, Isaacs C, et al.: Utilization of breast cancer screening in a clinically based sample of women after BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev 11 (10 Pt 1): 1115-8, 2002.
- Tinley ST, Houfek J, Watson P, et al.: Screening adherence in BRCA1/2 families is associated with primary physicians' behavior. Am J Med Genet A 125 (1): 5-11, 2004.
- Lerman C, Seay J, Balshem A, et al.: Interest in genetic testing among first-degree relatives of breast cancer patients. Am J Med Genet 57 (3): 385-92, 1995.
- Struewing JP, Lerman C, Kase RG, et al.: Anticipated uptake and impact of genetic testing in hereditary breast and ovarian cancer families. Cancer Epidemiol Biomarkers Prev 4 (2): 169-73, 1995.
- Jacobsen PB, Valdimarsdottier HB, Brown KL, et al.: Decision-making about genetic testing among women at familial risk for breast cancer. Psychosom Med 59 (5): 459-66, 1997 Sep-Oct.
- Watson M, Kash KM, Homewood J, et al.: Does genetic counseling have any impact on management of breast cancer risk? Genet Test 9 (2): 167-74, 2005.
Latest Updates to This Summary (03 / 06 / 2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Updated
This summary is written and maintained by the
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the genetics of breast and gynecologic cancers. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Genetics of Breast and Gynecologic Cancers are:
- Doreen Agnese, MD (The Ohio State University)
- Kathleen A. Calzone, PhD, RN, AGN-BC, FAAN (National Cancer Institute)
- Ilana Cass, MD (Dartmouth-Hitchcock Medical Center)
- Lee-may Chen, MD (UCSF Helen Diller Family Comprehensive Cancer Center)
- Mary B. Daly, MD, PhD (Fox Chase Cancer Center)
- Megan Frone, MS, CGC (National Cancer Institute)
- Jessica Hatton , MS, CGC (National Cancer Institute)
- Joanne Kotsopoulos, PhD (University of Toronto and Women's College Hospital)
- Suzanne C. O'Neill, PhD (Georgetown University)
- Tuya Pal, MD, FACMG, FCCMG (Vanderbilt-Ingram Cancer Center)
- John M. Quillin, PhD, MPH, MS (Virginia Commonwealth University)
- Padma Sheila Rajagopal, MD, MPH, MSC (National Cancer Institute)
- Charite Ricker, MS, CGC (University of Southern California)
- Catharine Wang, PhD, MSc (Boston University School of Public Health)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Cancer Genetics Editorial Board uses a
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Cancer Genetics Editorial Board. PDQ Genetics of Breast and Gynecologic Cancers. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at:
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in
Disclaimer
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our
Last Revised: 2025-03-06
This information does not replace the advice of a doctor. Ignite Healthwise, LLC, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.
Page Footer
I want to...
Audiences
Secure Member Sites
The Cigna Group Information
Disclaimer
Individual and family medical and dental insurance plans are insured by Cigna Health and Life Insurance Company (CHLIC), Cigna HealthCare of Arizona, Inc., Cigna HealthCare of Illinois, Inc., Cigna HealthCare of Georgia, Inc., Cigna HealthCare of North Carolina, Inc., Cigna HealthCare of South Carolina, Inc., and Cigna HealthCare of Texas, Inc. Group health insurance and health benefit plans are insured or administered by CHLIC, Connecticut General Life Insurance Company (CGLIC), or their affiliates (see
All insurance policies and group benefit plans contain exclusions and limitations. For availability, costs and complete details of coverage, contact a licensed agent or Cigna sales representative. This website is not intended for residents of New Mexico.