Shop for Plans
Shop for your own coverage
Plans through your employer
Learn about the medical, dental, pharmacy, behavioral, and voluntary benefits your employer may offer.
Learn
Living or working abroad?
Genetics of Gastric Cancer (PDQ®): Genetics - Health Professional Information [NCI]
Introduction
Gastric cancer is one of the most commonly diagnosed cancers in the world, with an incidence of over 1 million cases and an estimated 769,000 deaths occurring in 2020. Globally, this cancer ranked fifth for cancer incidence and fourth for cancer mortality in 2020.[
The genes that can predispose individuals to hereditary diffuse gastric cancer (HDGC) include the following:
-
CDH1 . -
CTNNA1 .
HDGC is the most common hereditary cancer syndrome that can predispose individuals to gastric cancer. This disease does not have an easily detectable precursor lesion. For more information, see
There are also hereditary cancer syndromes that can cause other forms of gastric cancer. These syndromes include the following:
- Lynch syndrome: Lynch syndrome is caused by pathogenic variants in MLH1, MSH2, MSH6, PMS2, and EPCAM. The amount of gastric cancer risk associated with Lynch syndrome and the role of gastric cancer screening remain unknown, especially since a Lynch syndrome–associated gastric cancer precursor has not yet been identified.[
6 ] - Familial adenomatous polyposis (FAP)/gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): FAP/GAPPS are caused by pathogenic variants in APC. Gastric cancer is typically preceded by fundic gland polyps.[
7 ] - Peutz-Jeghers syndrome (PJS): PJS is caused by pathogenic variants in STK11. Gastric cancer is typically preceded by hamartomatous polyps.[
8 ] - Juvenile polyposis syndrome (JPS): JPS is caused by pathogenic variants in SMAD4 and BMPR1A. Gastric cancer is typically preceded by hamartomatous polyps.
In each of these syndromes, gastric polyps and gastric cancer risk are of secondary importance to colorectal polyps and colorectal cancer. Gastric polyp burden (including polyp count and size) and gastric cancer risk may vary between individuals.
For more information on these genetic syndromes, see
Gastric Cancer Risk Factors
Environmental risk factors
Incidence rates for gastric cancer are the highest in Eastern Asia and Eastern Europe, while the rates in Northern America, Northern Europe, and Africa are generally low.[
Environmental risk factors for gastric cancer include the following:
1. Helicobacter pylori (H. pylori) infection.
- This may be most the impactful environmental risk factor for gastric cancer.
- An H. pylori infection may cause histological changes in the stomach, which can begin with chronic, active gastritis and can progress to gastric atrophy, intestinal metaplasia, gastric dysplasia, and eventually gastric cancer.[
9 ,10 ] - The combination of H. pylori and germline pathogenic variants in homologous recombination genes (ATM, BRCA1, BRCA2, and PALB2) increases the risk of gastric cancer in an additive manner. A study of 38,153 controls and 10,426 gastric cancer cases in Japan found that germline pathogenic variants in nine genes (APC, ATM, BRCA1, BRCA2, CDH1, MLH1, MSH2, MSH6, and PALB2) were associated with increased gastric cancer risk.[
11 ] The relative risk (RR) of gastric cancer in those with H. pylori and a pathogenic variant in one of four homologous recombination genes was 16.01 (confidence interval, 2.22–29.8). Risk persisted after adjusting for environmental risk factors like smoking, obesity, and salt intake. There was no increased risk in those who had H. pylori and a pathogenic variant in a mismatch repair gene. The authors noted that results primarily pertain to the Japanese population, since gastric cancer and H. pylori incidence rates are much higher in East Asian populations than in Western populations.
2. Diet.
- The regional variation in gastric cancer incidence is influenced by diet.[
12 ,13 ] - Gastric cancer risk may increase when individuals consume foods that are preserved with salt.[
13 ,14 ] - Low fruit intake and the consumption of a large amount of processed, grilled, or barbequed meats/fish may also increase gastric cancer risk.[
13 ]
3. Migrant effects.
- Gastric cancer risk may also be affected by migrant effects (i.e., effects incurred when individuals migrate from a region with high gastric cancer risk to a region with low gastric cancer risk or vice versa).
- Studies have demonstrated that the incidence of gastric cancer in immigrants correlates closely with the incidence of gastric cancer in their new host country after one or two generations.[
15 ,16 ,17 ]
4. Tobacco use.[
5. Alcohol use.[
6. Emerging evidence for obesity and gastroesophageal reflux disease (GERD).
- Emerging evidence suggests an association between increased gastric cancer risk, excess body weight, and tissue damage associated with GERD.[
18 ,19 ]
7. Racial and ethnic disparities.
- There are striking racial and ethnic disparities associated with gastric cancer incidence in the United States.[
20 ,21 ,22 ,23 ,24 ,25 ] - Studies that examine genetic and nongenetic risk factors, such as social determinants of health, are needed to understand and reduce the disparities in gastric cancer incidence that are seen in different racial and ethnic minority groups.
Intensive surveillance may be clinically warranted in individuals with hereditary, environmental, or ethnicity-based gastric cancer risk factors. However, more data are needed to show whether these risk factors increase or decrease gastric cancer risk in individuals with hereditary gastric cancer pathogenic variants. For more information about risk factors for gastric cancer in the general population, see
Familial risk factors
Individuals with family histories of gastric cancer have an increased risk of developing gastric cancer.[
Families with multiple gastric cancer cases have also been used to study the heritability of gastric cancer. A report in 1964 described a large, native Maori family from New Zealand.[
Precancerous Lesions
In the stomach, gastric adenocarcinoma can arise from visible lesions, from the histological progression of mucosal atrophy or, less commonly, from invisible lesions (i.e., submucosal infiltration, as seen in diffuse-type gastric adenocarcinoma). This section reviews both gastric polyps and the histological changes associated with chronic gastritis that can predispose an individual to gastric cancer.
Gastric polyps
Gastric polyps are common in the U.S. population and are found in up to 6% of individuals who undergo endoscopy. However, these polyps rarely progress to become gastric cancer.[
- Adenomatous.
- Hyperplastic.
- Inflammatory.
- Hamartomatous (associated with PJS, JPS, and PTEN hamartoma tumor syndromes [PHTS]).
- Fundic gland.[
42 ]
In general, the malignant potential of a polyp is determined by the polyp's histology, size, and degree of dysplasia, if present. Adenomatous and hyperplastic polyps have the highest malignant potentials, especially when H. pylori is present. In contrast, fundic gland polyps (with or without dysplasia) rarely progress to become gastric cancer, even in individuals who have FAP.[
Fundic gland polyps of the stomach are common and typically associated with long-term proton pump inhibitor (PPI) use.[
Inflammatory and hyperplastic polyps can present with many different pathological features. Unlike the colon, in which hyperplastic polyps carry little or no cancer risk, hyperplastic polyps of the stomach can enlarge and develop dysplastic foci.[
Hamartomatous polyps are seen in the stomachs of patients with PJS, JPS, and PHTS. For more information on these hereditary cancer syndromes, see
Gastric adenomas make up approximately 10% of all gastric polyps. A gastric adenoma's malignant potential is determined by its histological subtype and size.[
Chronic gastritis
Chronic gastritis, a histological diagnosis defined by the presence of a mononuclear cellular infiltrate in the lamina propria of the stomach, occurs due to environmental exposures or autoimmune gastritis. Chronic gastritis can lead to gastric intestinal metaplasia. H. pylori is the most common risk factor for intestinal metaplasia. Other risk factors for intestinal metaplasia include pernicious anemia, autoimmune gastritis, ethnicity, a family history of gastric cancer, dietary habits, smoking, and alcohol use.[
The steps of Correa's cascade include the following:
- Chronic gastritis (most commonly caused by H. pylori).
- Gastric mucosal atrophy.
- Gastric intestinal metaplasia.
- Gastric dysplasia (low-grade dysplasia followed by high-grade dysplasia).[
50 ,51 ]
Although these histological changes are associated with increased gastric cancer risk in the general population, the prevalence of findings and associated cancer risk in hereditary cancer syndromes is unknown. Each step in Correa's cascade only occurs in a minority of patients. There is considerable controversy regarding appropriate surveillance in patients with intestinal metaplasia.[
Endoscopic assessment
Gastric cancer screening is not routinely performed in the United States, where gastric cancer incidence is relatively low. Gastric cancer screening is also not usually performed in individuals with family histories of gastric cancer in which a hereditary gastric cancer pathogenic variant has not been identified. Large, prospective multicenter studies are needed to determine if gastric cancer screening programs would improve mortality in individuals from the United States with family histories of gastric cancer. In the United States, gastric cancer surveillance guidelines only exist for individuals with pathogenic variants in CDH1 or other gastric cancer risk genes (for more information, see
When evaluating any patient with hereditary gastric cancer risk, endoscopy includes assessment for gastric polyps and H. pylori —despite a lack of evidence demonstrating that H. pylori increases gastric cancer risk in the setting of all hereditary cancer syndromes.[
A small focus of intestinal metaplasia in the antrum is shown below. Intestinal metaplasia is not always visible on endoscopy. Instead, it is often found when taking random biopsies of the stomach.[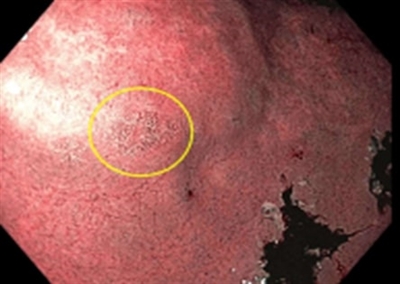
Endoscopic image showing a small area of intestinal metaplasia (circled in yellow) in the antrum of the stomach.
References:
- Allemani C, Matsuda T, Di Carlo V, et al.: Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391 (10125): 1023-1075, 2018.
- Sung H, Ferlay J, Siegel RL, et al.: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71 (3): 209-249, 2021.
- American Cancer Society: Cancer Facts and Figures 2024. American Cancer Society, 2024.
Available online . Last accessed December 30, 2024. - Uson PLS, Kunze KL, Golafshar MA, et al.: Germline Cancer Testing in Unselected Patients with Gastric and Esophageal Cancers: A Multi-center Prospective Study. Dig Dis Sci 67 (11): 5107-5115, 2022.
- Ku GY, Kemel Y, Maron SB, et al.: Prevalence of Germline Alterations on Targeted Tumor-Normal Sequencing of Esophagogastric Cancer. JAMA Netw Open 4 (7): e2114753, 2021.
- Møller P, Seppälä TT, Bernstein I, et al.: Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 67 (7): 1306-1316, 2018.
- Abraham SC, Nobukawa B, Giardiello FM, et al.: Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol 157 (3): 747-54, 2000.
- Giardiello FM, Brensinger JD, Tersmette AC, et al.: Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 119 (6): 1447-53, 2000.
- Correa P, Haenszel W, Cuello C, et al.: A model for gastric cancer epidemiology. Lancet 2 (7924): 58-60, 1975.
- Hooi JKY, Lai WY, Ng WK, et al.: Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 153 (2): 420-429, 2017.
- Usui Y, Taniyama Y, Endo M, et al.: Helicobacter pylori, Homologous-Recombination Genes, and Gastric Cancer. N Engl J Med 388 (13): 1181-1190, 2023.
- Tsugane S, Sasazuki S: Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer 10 (2): 75-83, 2007.
- Liu SJ, Huang PD, Xu JM, et al.: Diet and gastric cancer risk: an umbrella review of systematic reviews and meta-analyses of prospective cohort studies. J Cancer Res Clin Oncol 148 (8): 1855-1868, 2022.
- Song Y, Liu X, Cheng W, et al.: The global, regional and national burden of stomach cancer and its attributable risk factors from 1990 to 2019. Sci Rep 12 (1): 11542, 2022.
- Shimizu H, Mack TM, Ross RK, et al.: Cancer of the gastrointestinal tract among Japanese and white immigrants in Los Angeles County. J Natl Cancer Inst 78 (2): 223-8, 1987.
- Mousavi SM, Brandt A, Weires M, et al.: Cancer incidence among Iranian immigrants in Sweden and Iranian residents compared to the native Swedish population. Eur J Cancer 46 (3): 599-605, 2010.
- Lee J, Demissie K, Lu SE, et al.: Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control 14 (1): 78-85, 2007.
- Bouras E, Tsilidis KK, Triggi M, et al.: Diet and Risk of Gastric Cancer: An Umbrella Review. Nutrients 14 (9): , 2022.
- Poly TN, Lin MC, Syed-Abdul S, et al.: Proton Pump Inhibitor Use and Risk of Gastric Cancer: Current Evidence from Epidemiological Studies and Critical Appraisal. Cancers (Basel) 14 (13): , 2022.
- Shah SC, McKinley M, Gupta S, et al.: Population-Based Analysis of Differences in Gastric Cancer Incidence Among Races and Ethnicities in Individuals Age 50 Years and Older. Gastroenterology 159 (5): 1705-1714.e2, 2020.
- Anderson WF, Rabkin CS, Turner N, et al.: The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst 110 (6): 608-615, 2018.
- Islami F, DeSantis CE, Jemal A: Incidence Trends of Esophageal and Gastric Cancer Subtypes by Race, Ethnicity, and Age in the United States, 1997-2014. Clin Gastroenterol Hepatol 17 (3): 429-439, 2019.
- Holowatyj AN, Ulrich CM, Lewis MA: Racial/Ethnic Patterns of Young-Onset Noncardia Gastric Cancer. Cancer Prev Res (Phila) 12 (11): 771-780, 2019.
- De B, Rhome R, Jairam V, et al.: Gastric adenocarcinoma in young adult patients: patterns of care and survival in the United States. Gastric Cancer 21 (6): 889-899, 2018.
- Gupta S, Tao L, Murphy JD, et al.: Race/Ethnicity-, Socioeconomic Status-, and Anatomic Subsite-Specific Risks for Gastric Cancer. Gastroenterology 156 (1): 59-62.e4, 2019.
- Hemminki K, Sundquist J, Ji J: Familial risk for gastric carcinoma: an updated study from Sweden. Br J Cancer 96 (8): 1272-7, 2007.
- Yaghoobi M, Bijarchi R, Narod SA: Family history and the risk of gastric cancer. Br J Cancer 102 (2): 237-42, 2010.
- Lissowska J, Groves FD, Sobin LH, et al.: Family history and risk of stomach cancer in Warsaw, Poland. Eur J Cancer Prev 8 (3): 223-7, 1999.
- Palli D, Russo A, Ottini L, et al.: Red meat, family history, and increased risk of gastric cancer with microsatellite instability. Cancer Res 61 (14): 5415-9, 2001.
- Dhillon PK, Farrow DC, Vaughan TL, et al.: Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer 93 (1): 148-52, 2001.
- Minami Y, Tateno H: Associations between cigarette smoking and the risk of four leading cancers in Miyagi Prefecture, Japan: a multi-site case-control study. Cancer Sci 94 (6): 540-7, 2003.
- Eto K, Ohyama S, Yamaguchi T, et al.: Familial clustering in subgroups of gastric cancer stratified by histology, age group and location. Eur J Surg Oncol 32 (7): 743-8, 2006.
- Chen MJ, Wu DC, Ko YC, et al.: Personal history and family history as a predictor of gastric cardiac adenocarcinoma risk: a case-control study in Taiwan. Am J Gastroenterol 99 (7): 1250-7, 2004.
- Yatsuya H, Toyoshima H, Tamakoshi A, et al.: Individual and joint impact of family history and Helicobacter pylori infection on the risk of stomach cancer: a nested case-control study. Br J Cancer 91 (5): 929-34, 2004.
- Lichtenstein P, Holm NV, Verkasalo PK, et al.: Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343 (2): 78-85, 2000.
- JONES EG: FAMILIAL GASTRIC CANCER. N Z Med J 63: 287-96, 1964.
- Guilford P, Hopkins J, Harraway J, et al.: E-cadherin germline mutations in familial gastric cancer. Nature 392 (6674): 402-5, 1998.
- Guilford PJ, Hopkins JB, Grady WM, et al.: E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat 14 (3): 249-55, 1999.
- Corso G, Marrelli D, Pascale V, et al.: Frequency of CDH1 germline mutations in gastric carcinoma coming from high- and low-risk areas: metanalysis and systematic review of the literature. BMC Cancer 12: 8, 2012.
- Brooks-Wilson AR, Kaurah P, Suriano G, et al.: Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet 41 (7): 508-17, 2004.
- Carmack SW, Genta RM, Schuler CM, et al.: The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol 104 (6): 1524-32, 2009.
- Gullo I, Grillo F, Mastracci L, et al.: Precancerous lesions of the stomach, gastric cancer and hereditary gastric cancer syndromes. Pathologica 112 (3): 166-185, 2020.
- Mankaney G, Leone P, Cruise M, et al.: Gastric cancer in FAP: a concerning rise in incidence. Fam Cancer 16 (3): 371-376, 2017.
- Martin FC, Chenevix-Trench G, Yeomans ND: Systematic review with meta-analysis: fundic gland polyps and proton pump inhibitors. Aliment Pharmacol Ther 44 (9): 915-925, 2016.
- Jain R, Chetty R: Gastric hyperplastic polyps: a review. Dig Dis Sci 54 (9): 1839-46, 2009.
- Stolte M, Sticht T, Eidt S, et al.: Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy 26 (8): 659-65, 1994.
- Abraham SC, Park SJ, Lee JH, et al.: Genetic alterations in gastric adenomas of intestinal and foveolar phenotypes. Mod Pathol 16 (8): 786-95, 2003.
- Chen ZM, Scudiere JR, Abraham SC, et al.: Pyloric gland adenoma: an entity distinct from gastric foveolar type adenoma. Am J Surg Pathol 33 (2): 186-93, 2009.
- Altayar O, Davitkov P, Shah SC, et al.: AGA Technical Review on Gastric Intestinal Metaplasia-Epidemiology and Risk Factors. Gastroenterology 158 (3): 732-744.e16, 2020.
- Correa P: Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52 (24): 6735-40, 1992.
- Neumann WL, Coss E, Rugge M, et al.: Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol 10 (9): 529-41, 2013.
- Dinis-Ribeiro M, Kuipers EJ: How to Manage a Patient With Gastric Intestinal Metaplasia: An International Perspective. Gastroenterology 158 (6): 1534-1537, 2020.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2023. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2023.
Available with free registration. Last accessed June 28, 2023. - National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023.
Available online with free registration. Last accessed October 17, 2024. - Blair VR, McLeod M, Carneiro F, et al.: Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 21 (8): e386-e397, 2020.
Genetic Evaluation in Individuals With Personal and / or Family Histories of Gastric Cancer
Approximately 15% to 20% of gastric cancers may be caused by hereditary gastric cancer syndromes.[
The following items are important to elicit when collecting a patient's family history:
- Number of relatives with cancers on the same side of the family.
- Types of cancers seen in family members (including each cancer's histological subtype).a
- Ages of family members when they were diagnosed with cancer.
- Family members' gastrointestinal polyp histories (including each polyp's histological subtype).
- Number of family members with cleft lip or cleft palate (cleft lip or cleft palate has been linked to CDH1-associated hereditary gastric cancer).[
4 ]
a It is recommended that family history analysis focus on gastric cancer and other cancers (such as lobular breast cancer) that may be associated with hereditary diffuse gastric cancer (HDGC).
For information on collecting a family history, see the
In busy clinical settings, it might not be feasible to obtain a complete family history.[
Many factors can contribute to inaccurate reporting of a gastric cancer family history. In medicine, the stomach has a distinct definition and anatomical location in the body. The public's definition of the stomach is more ambiguous, which could lead to reporting inaccuracies.[
The poor validity of self-reported gastric cancer family histories needs to be considered during a patient's genetic evaluation and when creating an individual's clinical management recommendations. Studies have assessed the accuracy of self-reported family histories by comparing collected information with confirmation sources like death certificates, cancer registries, and histopathological records.[
Obtaining pathological documentation of personal/family histories of cancer and polyps is also integral when assessing a patient's hereditary gastric cancer risk. This documentation is especially important because genetic testing criteria has shifted over the years and has, in turn, broadened clinical criteria.[
The diagnosis of cancers before age 50 years is a hallmark feature of hereditary cancer syndromes. Attenuated forms of hereditary cancer syndromes also exist and are increasingly recognized. In these attenuated syndromes, the ages of gastric cancer onset can occur after age 50 years.[
Genetic Testing Approach
Patients who develop gastric cancer usually present at an advanced stage.[
Germline genetic testing criteria exists for the following genes associated with gastric cancer and/or gastric polyps: CDH1, CTNNA1, APC, STK11, SMAD4, and the Lynch syndrome genes (MLH1, MSH2, MSH6, EPCAM).[
When the patient has multiple cancers in the family that overlap with more than one hereditary cancer syndrome, another option is ordering multigene panel testing for all suspected genes. Multigene panel testing is the simultaneous testing of many genes for pathogenic and likely pathogenic variants, often at costs comparable to single-gene genetic testing. Hereditary cancer multigene panel testing includes cancer site-specific panels, high- or moderate-risk gene panels, guideline-based panels, and pancancer panels.[
For more information about selecting the appropriate genetic test, see the
Multigene panel testing implications
This section briefly summarizes the implications for patients undergoing multigene panel testing for hereditary gastric cancer syndromes. For more information about multigene panel testing, including genetic education, counseling considerations, and research, see the
Multigene panel testing can present challenges, including the unexpected discovery of pathogenic variants in genes that would not have been considered based on the patient's personal and/or family history. Pathogenic variants in many hereditary cancer genes can increase an individual's risk of developing more than one type of cancer. For instance, CDH1 pathogenic variants are associated with increased risk for both diffuse gastric cancer and lobular breast cancer.[
Cascade genetic testing
Cascade genetic testing identifies relatives who are at risk for a genetic condition by following the logical path of a pathogenic variant in a family. Cascade genetic testing is routinely offered to at-risk relatives once a pathogenic variant is identified. For more information, see the
References:
- Uson PLS, Kunze KL, Golafshar MA, et al.: Germline Cancer Testing in Unselected Patients with Gastric and Esophageal Cancers: A Multi-center Prospective Study. Dig Dis Sci 67 (11): 5107-5115, 2022.
- Ku GY, Kemel Y, Maron SB, et al.: Prevalence of Germline Alterations on Targeted Tumor-Normal Sequencing of Esophagogastric Cancer. JAMA Netw Open 4 (7): e2114753, 2021.
- Oliveira C, Pinheiro H, Figueiredo J, et al.: Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol 16 (2): e60-70, 2015.
- Frebourg T, Oliveira C, Hochain P, et al.: Cleft lip/palate and CDH1/E-cadherin mutations in families with hereditary diffuse gastric cancer. J Med Genet 43 (2): 138-42, 2006.
- Qureshi N, Wilson B, Santaguida P, et al.: Collection and use of cancer family history in primary care. Evid Rep Technol Assess (Full Rep) (159): 1-84, 2007.
- Mitchell RJ, Brewster D, Campbell H, et al.: Accuracy of reporting of family history of colorectal cancer. Gut 53 (2): 291-5, 2004.
- Douglas FS, O'Dair LC, Robinson M, et al.: The accuracy of diagnoses as reported in families with cancer: a retrospective study. J Med Genet 36 (4): 309-12, 1999.
- Ozanne EM, O'Connell A, Bouzan C, et al.: Bias in the reporting of family history: implications for clinical care. J Genet Couns 21 (4): 547-56, 2012.
- Mai PL, Garceau AO, Graubard BI, et al.: Confirmation of family cancer history reported in a population-based survey. J Natl Cancer Inst 103 (10): 788-97, 2011.
- Rashid A, Jagger C: Patients' knowledge of anatomical location of major organs within the human body: a comparison between Asians and non-Asians. Fam Pract 13 (5): 450-4, 1996.
- Fiederling J, Shams AZ, Haug U: Validity of self-reported family history of cancer: A systematic literature review on selected cancers. Int J Cancer 139 (7): 1449-60, 2016.
- Blair VR, McLeod M, Carneiro F, et al.: Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 21 (8): e386-e397, 2020.
- Colvin H, Yamamoto K, Wada N, et al.: Hereditary Gastric Cancer Syndromes. Surg Oncol Clin N Am 24 (4): 765-77, 2015.
- Xicola RM, Li S, Rodriguez N, et al.: Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet 56 (12): 838-843, 2019.
- National Cancer Institute: SEER Cancer Stat Facts: Stomach Cancer. Bethesda, Md: National Cancer Institute.
Available online . Last accessed February 21, 2025. - Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015.
- Domchek SM, Bradbury A, Garber JE, et al.: Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? J Clin Oncol 31 (10): 1267-70, 2013.
- Pharoah PD, Guilford P, Caldas C, et al.: Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 121 (6): 1348-53, 2001.
- Corso G, Intra M, Trentin C, et al.: CDH1 germline mutations and hereditary lobular breast cancer. Fam Cancer 15 (2): 215-9, 2016.
- van der Kaaij RT, van Kessel JP, van Dieren JM, et al.: Outcomes after prophylactic gastrectomy for hereditary diffuse gastric cancer. Br J Surg 105 (2): e176-e182, 2018.
- Lowstuter K, Espenschied CR, Sturgeon D, et al.: Unexpected CDH1 Mutations Identified on Multigene Panels Pose Clinical Management Challenges. JCO Precis Oncol 1: 1-12, 2017.
- Katona BW, Clark DF, Domchek SM: CDH1 on Multigene Panel Testing: Look Before You Leap. J Natl Cancer Inst 112 (4): 330-334, 2020.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023.
Available online with free registration. Last accessed October 17, 2024. - Hampel H: Genetic counseling and cascade genetic testing in Lynch syndrome. Fam Cancer 15 (3): 423-7, 2016.
Major Genes and Genetic Syndromes Associated With Gastric Cancer
The major heritable syndromes associated with increased gastric cancer risk are listed in
| Syndromea | Gene | Gastric Cancer Risk | Gastric Cancer Pathology | Gastric CancerSurveillance | Other Characteristics |
|---|---|---|---|---|---|
| CNS = central nervous system; CRC = colorectal cancer; EGD = esophagogastroduodenoscopy; GI = gastrointestinal; GIST = gastrointestinal stromal tumor. | |||||
| a All conditions are inherited in anautosomal dominantfashion, unless otherwise specified. | |||||
| b High-risk lesions include tubular adenomas, polyps with high-grade dysplasia, and pyloric gland adenomas. | |||||
| c Prophylactic total gastrectomy is not recommended prior to age 40 y. However, it may be considered for certain patients, especially those with a family histories of gastric cancer prior to age 25 y. | |||||
| |
CDH1andCTNNA1 | 33%–83%[ |
Diffuse type, signet ring cell carcinoma | EGD with multiple random biopsies of the stomach every 6–12 moor risk-reducing total gastrectomy between ages 18–40 yc[ |
For CDH1 only: Lobular breast cancer, cleft lip or cleft palate. It is currently unknown whetherCTNNA1is associated with lobular breast cancer or cleft lip or cleft palate |
| |
APC | 1.3%–5% in FAP and AFAP.[ |
Intestinal type | EGD for duodenal surveillance is also adequate surveillance for gastric cancers. Gastrectomy can be considered for patients with high-risk lesions that cannot be managed endoscopicallyb[ |
Gastric polyps (fundic gland polyps and adenomas), CRC, colorectal polyps (adenomas), desmoid tumors, duodenal tumors and cancer, thyroid cancer, brain cancer, ampullary cancer, pancreatic cancer, hepatoblastoma |
| |
APC | ||||
| |
APC(pathogenic variants/single nucleotide variantin the promoter 1B region ofAPCare associated with GAPPS-7) | In GAPPS, gastric cancer risk is significantly increased, but exact risk numbers are unknown[ |
|||
| |
MLH1,MSH2,MSH6,PMS2,EPCAM | 0.2%–9% | Intestinal type | Upper GI surveillance with EGD (preferably performed in conjunction with colonoscopy) starting at age 30–40 y (or earlier based on the patient's family history). Repeat EGD every 2–4 y[ |
CRC, endometrial cancer, ovarian cancer, breast cancer, pancreatic cancer, bladder cancer, biliary tract cancer, urothelial cancer, small bowel cancer, prostate cancer, brain/CNS tumor |
| |
STK11(also known asLKB1) | 29%[ |
Intestinal type (arising from dysplasia in hamartomas) | EGD every 2–3 y beginning in the late adolescence[ |
Hamartomatous GI polyps; breast cancer; CRC; small bowel cancer; pancreatic cancer; Sertoli cell tumors of the ovary and testes; cervical adenoma malignum; endometrial cancer; lung cancer; mucocutaneous hyperpigmentation of the mouth, lips, nose, eyes, genitalia and/or fingers |
| |
SMAD4,BMPR1A | 21% if multiple polyps are present | Intestinal type (arising from dysplasia in juvenile polyps/hamartomas) | EGD every 2–3 y. If polyps are found, conduct EGD at shorter intervals based on polyp size, number, and pathology[ |
Juvenile/hamartomatous colorectal polyps, CRC, hereditary hemorrhagic telangiectasia |
| |
TP53 | 2.8% | Possibly intestinal type | Breast cancer, adrenocortical carcinoma, sarcoma, brain/CNS tumor, leukemia | |
References:
- Roberts ME, Ranola JMO, Marshall ML, et al.: Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol 5 (9): 1325-1331, 2019.
- Pharoah PD, Guilford P, Caldas C, et al.: Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 121 (6): 1348-53, 2001.
- Hansford S, Kaurah P, Li-Chang H, et al.: Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol 1 (1): 23-32, 2015.
- Kaurah P, MacMillan A, Boyd N, et al.: Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 297 (21): 2360-72, 2007.
- Xicola RM, Li S, Rodriguez N, et al.: Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet 56 (12): 838-843, 2019.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer. Version 1.2023. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023.
Available online with free registration. Last accessed October 17, 2024. - Cannon AR, Keener M, Neklason D, et al.: Surgical Interventions, Malignancies, and Causes of Death in a FAP Patient Registry. J Gastrointest Surg 25 (2): 452-456, 2021.
- Mankaney G, Leone P, Cruise M, et al.: Gastric cancer in FAP: a concerning rise in incidence. Fam Cancer 16 (3): 371-376, 2017.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2023. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2023.
Available with free registration. Last accessed June 28, 2023. - Li J, Woods SL, Healey S, et al.: Point Mutations in Exon 1B of APC Reveal Gastric Adenocarcinoma and Proximal Polyposis of the Stomach as a Familial Adenomatous Polyposis Variant. Am J Hum Genet 98 (5): 830-842, 2016.
Other Genes Associated With Gastric Cancer
Preliminary evidence has linked gastric cancer to other genes that are not typically associated with this type of cancer, such as ATM, BRCA1, BRCA2, BRIP1, PALB2, and RAD51D. Studies suggest a possible association between gastric cancer risk and pathogenic variants in both established gastric cancer genes and in moderate- and high-risk genes that are not typically associated with gastric cancer. However, clinical practice changes are not indicated based on this preliminary evidence.
Several studies searched for candidate genes in hereditary gastric cancer cases that were not associated with CDH1 pathogenic variants. Some of these cases may be explained by pathogenic variants in homologous recombination (HR) DNA repair genes, particularly PALB2:
- One of the first studies to identify variants that may increase gastric cancer risk performed whole-exome sequencing (WES) in 28 patients and sequenced candidate genes in 333 patients.[
1 ] In 11 cases, pathogenic variants were identified in genes that regulate HR, including eight PALB2 pathogenic variants, two BRCA1 pathogenic variants, and one RAD51C pathogenic variant. WES in four tumor samples (from three PALB2 carriers and one RAD51C carrier) did not find a loss of heterozygosity (LOH). However, all of the tumors (4/4) were enriched for a mutational signature that is characteristic of HR defects, suggesting a causal link between these genes and gastric cancer risk. - Another study of 28 CDH1-negative individuals with clinical diagnoses of hereditary diffuse gastric cancer used WES.[
2 ] A PALB2 loss-of-function variant was found in one patient with a history of gastric cancer and breast cancer. Two pathogenic variants were also identified in MSH2 (mismatch repair gene), and two variants were identified in RECQL5 (DNA repair gene). - In a large investigation of cancer predisposition variants, 5 of 443 patients with gastric cancer carried PALB2 loss-of-function variants with variable LOH (2 of 5 tumors), similar to that seen in other PALB2-associated tumors.[
3 ] - However, a study of 58 patients who met clinical criteria for hereditary diffuse gastric cancer but tested negative for CDH1 pathogenic variants reported conflicting findings.[
4 ] Unlike similar studies, PALB2 sequencing did not discover pathogenic variants in these patients.
In summary, these findings suggest that defects in HR genes increase risk for gastric cancer and highlight a role for the known cancer predisposition gene, PALB2 as a potential candidate gene for hereditary gastric cancer. However, case-control studies are needed to confirm whether PALB2 is associated with hereditary gastric cancer susceptibility. Gastric cancer screening is not indicated for PALB2 carriers.
Additional studies investigated potential candidate genes for gastric cancer:
- Fifty-one adults with gastric cancer (with any type of histology) were part of an international hereditary cancer research network. These individuals were retrospectively selected to undergo germline genetic testing with a 706–candidate gene genetic testing panel.[
5 ] Twenty pathogenic or likely pathogenic variants were identified in 18 participants (35%). Some of these pathogenic variants were in genes not typically associated with gastric cancer, including ATM, BRCA2, BRIP1, FANCC, and TP53. - Another study of 34 patients with gastric cancer, who were not selected based on their clinical characteristics or family histories of cancer, found a pathogenic variant rate of 17.6% on a genetic testing panel that examined between 83 and 84 genes.[
6 ] Two pathogenic variants were identified in BRCA1, and one pathogenic variant was identified in each of the following genes: BRCA2, CDH1, FH, and SDHA. - A cross-sectional study performed at a tertiary cancer center assessed the presence of pathogenic variants on a genetic testing panel that examined 76 or 88 genes in 243 patients with gastric cancer who consented to paired somatic and germline sequencing.[
7 ] A total of 48 patients with gastric cancer (19.8%) had germline pathogenic variants. In high- and moderate-penetrance genes that are not classically associated with gastric cancer, pathogenic variants were found most frequently in BRCA1, BRCA2, and ATM. - One of the largest studies included 282 patients with gastric cancer who were of Han Chinese descent.[
8 ] These patients were enrolled at medical centers in Beijing and underwent a 69–cancer gene panel, which revealed that 7.1% of patients had pathogenic variants. Pathogenic variants were found most often in ATM (detection rate, 1.1% [3/282 patients]), followed by BRCA1, BRIP1, and RAD51D, which all had a detection rate of 0.7% (2/282 patients).
Additional studies are warranted to determine if there is a causal relationship between these genes and gastric cancer risk. However, the rate of pathogenic variants found in these studies supports the use of multigene panels in patients with gastric cancer undergoing germline genetic testing. For more information, see the
References:
- Sahasrabudhe R, Lott P, Bohorquez M, et al.: Germline Mutations in PALB2, BRCA1, and RAD51C, Which Regulate DNA Recombination Repair, in Patients With Gastric Cancer. Gastroenterology 152 (5): 983-986.e6, 2017.
- Fewings E, Larionov A, Redman J, et al.: Germline pathogenic variants in PALB2 and other cancer-predisposing genes in families with hereditary diffuse gastric cancer without CDH1 mutation: a whole-exome sequencing study. Lancet Gastroenterol Hepatol 3 (7): 489-498, 2018.
- Huang KL, Mashl RJ, Wu Y, et al.: Pathogenic Germline Variants in 10,389 Adult Cancers. Cell 173 (2): 355-370.e14, 2018.
- Carreño M, Pena-Couso L, Mercadillo F, et al.: Investigation on the Role of PALB2 Gene in CDH1-Negative Patients With Hereditary Diffuse Gastric Cancer. Clin Transl Gastroenterol 11 (12): e00280, 2020.
- Slavin T, Neuhausen SL, Rybak C, et al.: Genetic Gastric Cancer Susceptibility in the International Clinical Cancer Genomics Community Research Network. Cancer Genet 216-217: 111-119, 2017.
- Uson PLS, Kunze KL, Golafshar MA, et al.: Germline Cancer Testing in Unselected Patients with Gastric and Esophageal Cancers: A Multi-center Prospective Study. Dig Dis Sci 67 (11): 5107-5115, 2022.
- Ku GY, Kemel Y, Maron SB, et al.: Prevalence of Germline Alterations on Targeted Tumor-Normal Sequencing of Esophagogastric Cancer. JAMA Netw Open 4 (7): e2114753, 2021.
- Ji K, Ao S, He L, et al.: Characteristics of cancer susceptibility genes mutations in 282 patients with gastric adenocarcinoma. Chin J Cancer Res 32 (4): 508-515, 2020.
Latest Updates to This Summary (01 / 03 / 2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the genetics of gastric cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Genetics of Gastric Cancer are:
- Grace-Ann O. Fasaye, ScM, CGC (National Cancer Institute)
- Gautam Mankaney, MD (Virginia Mason Franciscan Health)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Cancer Genetics Editorial Board uses a
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Cancer Genetics Editorial Board. PDQ Genetics of Gastric Cancer. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at:
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in
Disclaimer
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our
Last Revised: 2025-01-03
This information does not replace the advice of a doctor. Ignite Healthwise, LLC, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.
Page Footer
I want to...
Audiences
Secure Member Sites
The Cigna Group Information
Disclaimer
Individual and family medical and dental insurance plans are insured by Cigna Health and Life Insurance Company (CHLIC), Cigna HealthCare of Arizona, Inc., Cigna HealthCare of Illinois, Inc., Cigna HealthCare of Georgia, Inc., Cigna HealthCare of North Carolina, Inc., Cigna HealthCare of South Carolina, Inc., and Cigna HealthCare of Texas, Inc. Group health insurance and health benefit plans are insured or administered by CHLIC, Connecticut General Life Insurance Company (CGLIC), or their affiliates (see
All insurance policies and group benefit plans contain exclusions and limitations. For availability, costs and complete details of coverage, contact a licensed agent or Cigna sales representative. This website is not intended for residents of New Mexico.