Treatment Option Overview
There are different types of treatment for patients with myelodysplastic/myeloproliferative neoplasms.
Different types of treatments are available for patients with myelodysplastic/myeloproliferative neoplasms. Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
The following types of treatment are used:
Watchful waiting
Watchful waiting is closely monitoring a patient's condition without giving any treatment until signs or symptoms appear or change. It is sometimes used to treat chronic myelomonocytic leukemia in patients with no or mild symptoms.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy). Combination chemotherapy is treatment using more than one anticancer drug.
For more information, see Drugs Approved for Myeloproliferative Neoplasms or Myelodysplastic Syndromes.
Other drug therapy
13-cis retinoic acid is a vitamin -like drug that slows the cancer's ability to make more cancer cells and changes the way these cells look and act.
Stem cell transplant
Chemotherapy is given to kill abnormal cells or cancer cells. Healthy cells, including blood -forming cells, are also destroyed by the cancer treatment. Stem cell transplant is a treatment to replace the blood-forming cells. Stem cells (immature blood cells) are removed from the blood or bone marrow of the patient or a donor and are frozen and stored. After the patient completes chemotherapy, the stored stem cells are thawed and given back to the patient through an infusion. These reinfused stem cells grow into (and restore) the body's blood cells. 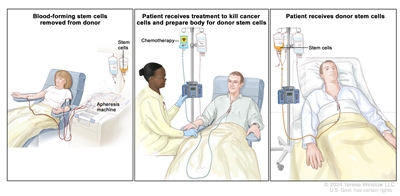
Donor stem cell transplant. (Step 1): Four to five days before donor stem cell collection, the donor receives a medicine to increase the number of stem cells circulating through their bloodstream (not shown). The blood-forming stem cells are then collected from the donor through a large vein in their arm. The blood flows through an apheresis machine that removes the stem cells. The rest of the blood is returned to the donor through a vein in their other arm. (Step 2): The patient receives chemotherapy to kill cancer cells and prepare their body for the donor stem cells. The patient may also receive radiation therapy (not shown). (Step 3): The patient receives an infusion of the donor stem cells.
Supportive care
Supportive care is given to lessen the problems caused by the disease or its treatment. Supportive care may include transfusion therapy or drug therapy, such as antibiotics to fight infection.
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells.
- Tyrosine kinase inhibitor (TKI) therapy: TKI therapy blocks signals that tumors need to grow. TKIs block the enzyme tyrosine kinase that causes stem cells to become more blood cells (blasts) than the body needs. Imatinib mesylate (Gleevec) is used to treat myelodysplastic/myeloproliferative neoplasm, unclassifiable.
For more information, see Drugs Approved for Myeloproliferative Neoplasms or Myelodysplastic Syndromes.
New types of treatment are being tested in clinical trials.
For some people, joining a clinical trial may be an option. There are different types of clinical trials for people with cancer. For example, a treatment trial tests new treatments or new ways of using current treatments. Supportive care and palliative care trials look at ways to improve quality of life, especially for those who have side effects from cancer and its treatment.
You can use the clinical trial search to find NCI-supported cancer clinical trials accepting participants. The search allows you to filter trials based on the type of cancer, your age, and where the trials are being done. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Learn more about clinical trials, including how to find and join one, at Clinical Trials Information for Patients and Caregivers.
Treatment for myelodysplastic/myeloproliferative neoplasms may cause side effects.
For information about side effects caused by treatment for cancer, visit our Side Effects page.
Follow-up care may be needed.
As you go through treatment, you will have follow-up tests or check-ups. Some tests that were done to diagnose or stage the cancer may be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back).