Shop for Plans
Shop for your own coverage
Plans through your employer
Learn about the medical, dental, pharmacy, behavioral, and voluntary benefits your employer may offer.
Learn
Living or working abroad?
Pancreatic Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]
General Information About Pancreatic Cancer
This summary provides information about the treatment of exocrine pancreatic cancer.
Incidence and Mortality
Estimated new cases and deaths from pancreatic cancer in the United States in 2025:[
- New cases: 67,440.
- Deaths: 51,980.
The incidence of pancreatic cancer has markedly increased over the past several decades. In the United States, it ranks as the fourth leading cause of cancer death in men and the third leading cause of cancer death in women.[
Risk Factors
Risk factors for development of pancreatic cancer include:[
- A family history of pancreatic cancer.
- Cigarette smoking.
- Obesity.
- Chronic pancreatitis.
- Certain genetic disorders (such as those associated with the BRCA1, BRCA2, PALB2, and ATM genes).
Anatomy
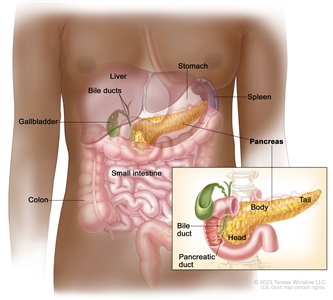
Anatomy of the pancreas.
Cancers of the pancreas are commonly identified by the site of involvement within the pancreas. Surgical approaches differ for masses in the head, body, tail, or uncinate process of the pancreas.
Clinical Features
Pancreatic cancer symptoms depend on the site of the tumor within the pancreas and the degree of tumor involvement.
In the early stages of pancreatic cancer, there are not many noticeable symptoms. As the cancer grows, symptoms may include:
- Jaundice.
- Light-colored stools or dark urine.
- Pain in the upper or middle abdomen and back.
- Weight loss for no known reason.
- Loss of appetite.
- Fatigue.
Diagnostic and Staging Evaluation
Pancreatic cancer is difficult to detect and diagnose for the following reasons:
- There are no noticeable signs or symptoms in the early stages of pancreatic cancer.
- The signs of pancreatic cancer, when present, are like the signs of many other illnesses, such as pancreatitis or an ulcer.
- The pancreas is obscured by other organs in the abdomen and is difficult to visualize clearly on imaging tests.
To appropriately treat pancreatic cancer, it is crucial to evaluate whether the cancer can be resected.
Imaging
Imaging tests may help diagnose pancreatic cancer and identify patients with disease that is not amenable to resection. Imaging tests may include:[
- Helical computed tomographic scan.
- Magnetic resonance imaging scan.
- Endoscopic ultrasonography.
- Minimally invasive techniques, such as laparoscopy and laparoscopic ultrasonography, which may be used to decrease the use of laparotomy.[
5 ,6 ]
Peritoneal cytology
In a case series of 228 patients, positive peritoneal cytology had a positive predictive value of 94%, specificity of 98%, and sensitivity of 25% for determining unresectability.[
Tumor markers
No tumor-specific markers exist for pancreatic cancer. Markers such as serum cancer antigen (CA) 19-9 have low specificity. Most patients with pancreatic cancer have an elevated CA 19-9 level at diagnosis. Increased CA 19-9 levels during or after definitive therapy may identify patients with progressive tumor growth.[
Prognosis and Survival
The primary factors that influence prognosis are:
- Whether the tumor is localized and can be completely resected.
- Whether the tumor has spread to lymph nodes or elsewhere.
Exocrine pancreatic cancer is rarely curable and has an overall survival (OS) rate of less than 6%.[
The highest cure rate occurs when the tumor is truly localized to the pancreas; however, this stage of disease accounts for less than 20% of cases. For patients with localized disease and small cancers (<2 cm) with no lymph node metastases and no extension beyond the capsule of the pancreas, complete surgical resection is associated with an actuarial 5-year survival rate of 18% to 24%.[
Surgical resection is the mainstay of curative treatment and provides a survival benefit in patients with small, localized pancreatic tumors, but it should be considered only alongside systemic therapy. Patients with unresectable, metastatic, or recurrent disease are unlikely to benefit from surgical resection.
Patients with any stage of pancreatic cancer are candidates for clinical trials because of the poor response to chemotherapy, radiation therapy, and surgery as conventionally used.
Information about ongoing clinical trials for pancreatic cancer is available from the
Palliative Therapy
Palliation of symptoms may be achieved with conventional treatment (systemic chemotherapy).
Palliative measures that may improve quality of life without affecting OS include:[
- Surgical or radiological biliary decompression.
- Relief of gastric outlet obstruction.
- Pain control.
- Psychological care to address the potentially disabling psychological events associated with the diagnosis and treatment of pancreatic cancer.[
13 ]
References:
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025.
Available online . Last accessed January 16, 2025. - Tersmette AC, Petersen GM, Offerhaus GJ, et al.: Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res 7 (3): 738-44, 2001.
- Nöthlings U, Wilkens LR, Murphy SP, et al.: Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst 97 (19): 1458-65, 2005.
- Riker A, Libutti SK, Bartlett DL: Advances in the early detection, diagnosis, and staging of pancreatic cancer. Surg Oncol 6 (3): 157-69, 1997.
- John TG, Greig JD, Carter DC, et al.: Carcinoma of the pancreatic head and periampullary region. Tumor staging with laparoscopy and laparoscopic ultrasonography. Ann Surg 221 (2): 156-64, 1995.
- Minnard EA, Conlon KC, Hoos A, et al.: Laparoscopic ultrasound enhances standard laparoscopy in the staging of pancreatic cancer. Ann Surg 228 (2): 182-7, 1998.
- Merchant NB, Conlon KC, Saigo P, et al.: Positive peritoneal cytology predicts unresectability of pancreatic adenocarcinoma. J Am Coll Surg 188 (4): 421-6, 1999.
- Willett CG, Daly WJ, Warshaw AL: CA 19-9 is an index of response to neoadjunctive chemoradiation therapy in pancreatic cancer. Am J Surg 172 (4): 350-2, 1996.
- Siegel R, Naishadham D, Jemal A: Cancer statistics, 2013. CA Cancer J Clin 63 (1): 11-30, 2013.
- Yeo CJ, Abrams RA, Grochow LB, et al.: Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 225 (5): 621-33; discussion 633-6, 1997.
- Sohn TA, Lillemoe KD, Cameron JL, et al.: Surgical palliation of unresectable periampullary adenocarcinoma in the 1990s. J Am Coll Surg 188 (6): 658-66; discussion 666-9, 1999.
- Baron TH: Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 344 (22): 1681-7, 2001.
- Passik SD, Breitbart WS: Depression in patients with pancreatic carcinoma. Diagnostic and treatment issues. Cancer 78 (3 Suppl): 615-26, 1996.
Cellular Classification of Pancreatic Cancer
Pancreatic cancer includes the following carcinomas:
Malignant
- Duct cell carcinoma (90% of all cases).
- Acinar cell carcinoma.
- Adenosquamous carcinoma.
- Cystadenocarcinoma (serous and mucinous types).
- Giant cell carcinoma.
- Invasive adenocarcinoma associated with cystic mucinous neoplasm or intraductal papillary mucinous neoplasm.
- Mixed type (ductal-endocrine or acinar-endocrine).
- Mucinous carcinoma.
- Pancreatoblastoma.
- Papillary-cystic neoplasm (Frantz tumor). This tumor has lower malignant potential and may be cured with surgery alone.[
1 ,2 ] - Papillary mucinous carcinoma.
- Signet ring carcinoma.
- Small cell carcinoma.
- Unclassified.
- Undifferentiated carcinoma.
Borderline Malignancies
- Intraductal papillary mucinous tumor with dysplasia.[
3 ] - Mucinous cystic tumor with dysplasia.
- Pseudopapillary solid tumor.
References:
- Sanchez JA, Newman KD, Eichelberger MR, et al.: The papillary-cystic neoplasm of the pancreas. An increasingly recognized clinicopathologic entity. Arch Surg 125 (11): 1502-5, 1990.
- Warshaw AL, Compton CC, Lewandrowski K, et al.: Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg 212 (4): 432-43; discussion 444-5, 1990.
- Sohn TA, Yeo CJ, Cameron JL, et al.: Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg 234 (3): 313-21; discussion 321-2, 2001.
Stage Information for Pancreatic Cancer
The staging system for pancreatic exocrine cancer continues to evolve. Clinical staging is guided by resectability, which is strongly influenced by surgical judgment. Consensus guidelines for surgical resectability (e.g., National Comprehensive Cancer Network, MD Anderson Cancer Center, American Hepato-Pancreato-Biliary Association, and International Hepato-Pancreato-Biliary Association) continue to be refined, but are traditionally stratified by the following tumor characteristics:
- Resectable: tumors without vascular involvement.
- Borderline resectable: tumors with involvement of vasculature, involvement of local structures, or other evidence of a high risk of R1 resection.
- Locally advanced: tumors with local invasion (primarily vascular involvement) that preclude surgical intervention.
- Metastatic: cancer that has spread beyond the primary pancreatic tumor to other organs.
The American Joint Committee on Cancer (AJCC) has designated staging by TNM (tumor, node, metastasis) classification.[
AJCC Stage Groupings and TNM Definitions
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| a Reprinted with permission from AJCC: Exocrine Pancreas. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 337–47. | |||
| 0 | Tis, N0, M0 | Tis = Carcinomain situ. This includes high-grade pancreatic intraepithelial neoplasia (PanIn-3), intraductal papillary mucinous neoplasm with high-grade dysplasia, intraductal tubulopapillary neoplasm with high-grade dysplasia, and mucinous cystic neoplasm with high-grade dysplasia. | 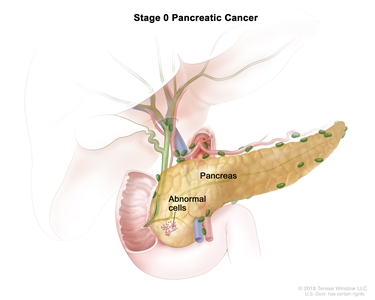 |
| N0 = No regional lymph node metastases. | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| a Reprinted with permission from AJCC: Exocrine Pancreas. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 337–47. | |||
| IA | T1, N0, M0 | T1 = Tumor ≤2 cm in greatest dimension. | 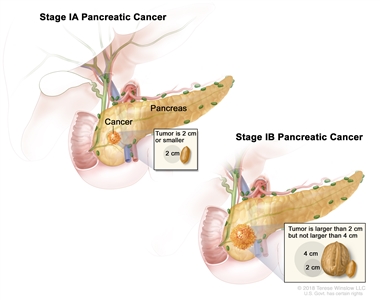 |
| –T1a = Tumor ≤0.5 cm in greatest dimension. | |||
| –T1b = Tumor >0.5 cm and <1 cm in greatest dimension. | |||
| –T1c = Tumor 1–2 cm in greatest dimension. | |||
| N0 = No regional lymph node metastases. | |||
| M0 = No distant metastasis. | |||
| IB | T2, N0, M0 | T2 = Tumor >2 cm and ≤4 cm in greatest dimension. | |
| N0 = No regional lymph node metastases. | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| a Reprinted with permission from AJCC: Exocrine Pancreas. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 337–47. | |||
| IIA | T3, N0, M0 | T3 = Tumor >4 cm in greatest dimension. |  |
| N0 = No regional lymph node metastases. | |||
| M0 = No distant metastasis. | |||
| IIB | T1, N1, M0 | T1 = Tumor ≤2 cm in greatest dimension. | 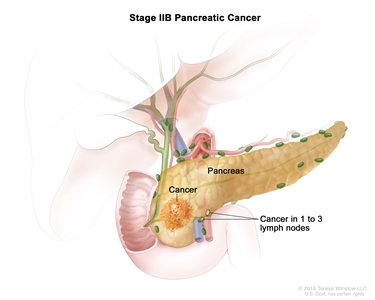 |
| –T1a = Tumor ≤0.5 cm in greatest dimension. | |||
| –T1b = Tumor >0.5 cm and <1 cm in greatest dimension. | |||
| –T1c = Tumor 1–2 cm in greatest dimension. | |||
| N1 = Metastasis in one to three regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T2, N1, M0 | T2 = Tumor >2 cm and ≤4 cm in greatest dimension. | ||
| N1 = Metastasis in one to three regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T3, N1, M0 | T3 = Tumor >4 cm in greatest dimension. | ||
| N1 = Metastasis in one to three regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| a Reprinted with permission from AJCC: Exocrine Pancreas. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 337–47. | |||
| III | T1, N2, M0 | T1 = Tumor ≤2 cm in greatest dimension. |  |
| –T1a = Tumor ≤0.5 cm in greatest dimension. | |||
| –T1b = Tumor >0.5 cm and <1 cm in greatest dimension. | |||
| –T1c = Tumor 1–2 cm in greatest dimension. | |||
| N2 = Metastasis in four or more regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T2, N2, M0 | T2 = Tumor >2 cm and ≤4 cm in greatest dimension. | ||
| N2 = Metastasis in four or more regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T3, N2, M0 | T3 = Tumor >4 cm in greatest dimension. | ||
| N2 = Metastasis in four or more regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T4, Any N, M0 | T4 = Tumor involves celiac axis, superior mesenteric artery, and/or common hepatic artery, regardless of size. | ||
| NX = Regional lymph nodes cannot be assessed. | |||
| N0 = No regional lymph node metastases. | |||
| N1 = Metastasis in one to three regional lymph nodes. | |||
| N2 = Metastasis in four or more regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| Stage | TNM | Description | Illustration |
|---|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | |||
| a Reprinted with permission from AJCC: Exocrine Pancreas. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 337–47. | |||
| IV | Any T, Any N, M1 | TX = Primary tumor cannot be assessed. | 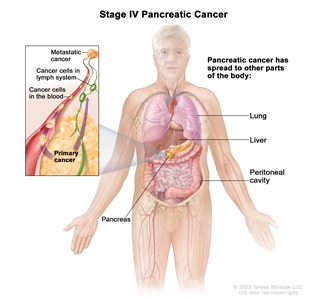 |
| T0 = No evidence of primary tumor. | |||
| Tis = Carcinomain situ. This includes high-grade pancreatic intraepithelial neoplasia (PanIn-3), intraductal papillary mucinous neoplasm with high-grade dysplasia, intraductal tubulopapillary neoplasm with high-grade dysplasia, and mucinous cystic neoplasm with high-grade dysplasia. | |||
| T1 = Tumor ≤2 cm in greatest dimension. | |||
| –T1a = Tumor ≤0.5 cm in greatest dimension. | |||
| –T1b = Tumor >0.5 cm and <1 cm in greatest dimension. | |||
| –T1c = Tumor 1–2 cm in greatest dimension. | |||
| T2 = Tumor >2 cm and ≤4 cm in greatest dimension. | |||
| T3 = Tumor >4 cm in greatest dimension. | |||
| T4 = Tumor involves celiac axis, superior mesenteric artery, and/or common hepatic artery, regardless of size. | |||
| NX = Regional lymph nodes cannot be assessed. | |||
| N0 = No regional lymph node metastases. | |||
| N1 = Metastasis in one to three regional lymph nodes. | |||
| N2 = Metastasis in four or more regional lymph nodes. | |||
| M1 = Distant metastasis. | |||
References:
- Kakar S, Pawlik TM, Allen PJ: Exocrine Pancreas. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 337–47.
Treatment Option Overview for Pancreatic Cancer
Surgical resection, when feasible, remains the primary treatment modality for patients with pancreatic cancer. On occasion, resection can lead to long-term survival, and it provides effective palliation.[
Postoperative chemotherapy improves overall survival, but the role of chemoradiation remains controversial.
Complications of pancreatic cancer include:
- Malabsorption: Frequently, malabsorption caused by exocrine insufficiency contributes to malnutrition. Pancreatic enzyme replacement can help alleviate this problem.
- Pain: Celiac axis and intrapleural nerve blocks can provide highly effective and long-lasting control of pain for some patients. For more information, see
Cancer Pain .
The survival rate of patients with any stage of pancreatic exocrine cancer is poor. Clinical trials are appropriate for patients with any stage of disease and should be considered before palliative approaches are selected.
Information about ongoing clinical trials for pancreatic cancer is available from the
| |
Treatment Options |
|---|---|
| Resectable or borderline resectable pancreatic cancer | |
| |
|
| |
|
| |
|
| Preoperative chemotherapy and/or radiation therapy (under clinical evaluation) | |
| Alternative radiation techniques (under clinical evaluation) | |
| Locally advanced pancreatic cancer | |
| |
|
| |
|
| |
|
| Clinical trials evaluating novel agents in combination with chemotherapy or chemoradiation therapy for patients with unresectable tumors | |
| Intraoperative radiation therapy and/or implantation of radioactive sources (under clinical evaluation) | |
| Metastatic or recurrent pancreatic cancer | |
| Clinical trials evaluating new anticancer agents alone or in combination with chemotherapy |
Palliative therapies can be considered in patients with any stage of disease. For more information, see the
Capecitabine and Fluorouracil Dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[
References:
- Yeo CJ, Cameron JL, Lillemoe KD, et al.: Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 221 (6): 721-31; discussion 731-3, 1995.
- Conlon KC, Klimstra DS, Brennan MF: Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 223 (3): 273-9, 1996.
- Yeo CJ, Abrams RA, Grochow LB, et al.: Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 225 (5): 621-33; discussion 633-6, 1997.
- Lidsky ME, Sun Z, Nussbaum DP, et al.: Going the Extra Mile: Improved Survival for Pancreatic Cancer Patients Traveling to High-volume Centers. Ann Surg 266 (2): 333-338, 2017.
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021.
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016.
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021.
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018.
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018.
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022.
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022.
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023.
Treatment of Resectable or Borderline Resectable Pancreatic Cancer
Treatment Options for Resectable or Borderline Resectable Pancreatic Cancer
Treatment options for resectable or borderline resectable pancreatic cancer include:
-
Neoadjuvant therapy : Neoadjuvant chemotherapy with or without chemoradiation therapy. -
Surgery : Radical pancreatic resection including:- Whipple procedure (pancreaticoduodenal resection).
- Total pancreatectomy when necessary for adequate margins.
- Distal pancreatectomy for tumors of the body and tail of the pancreas.[
1 ,2 ]
-
Postoperative chemotherapy : Radical pancreatic resection followed by chemotherapy.[3 ] -
Postoperative chemoradiation therapy : Radical pancreatic resection followed by fluorouracil (5-FU) chemotherapy and radiation therapy.[4 ,5 ,6 ,7 ,8 ] - Preoperative chemotherapy and/or radiation therapy (under clinical evaluation).
- Alternative radiation techniques (under clinical evaluation).
Palliative therapies can be considered in patients with any stage of disease. For more information, see the
Neoadjuvant therapy
Neoadjuvant therapy is chemotherapy with or without chemoradiation therapy given before surgery. The role of neoadjuvant therapy has been evaluated in retrospective studies (Surveillance, Epidemiology, and End Results [SEER] Program database and National Cancer Database) and is recommended by multiple consensus guidelines for the management of patients with borderline resectable pancreatic cancer. It is being evaluated in patients with resectable or borderline resectable pancreatic cancer in several ongoing trials.[
Evidence (neoadjuvant chemotherapy with or without chemoradiation therapy):
- The phase II, multicenter, randomized A021501 trial (NCT02839343) enrolled 126 patients with borderline resectable pancreatic cancer from institutions in the National Clinical Trials Network cooperative groups between 2017 and 2019. Patients were assigned to receive either eight 2-week cycles of modified FOLFIRINOX (oxaliplatin, leucovorin, irinotecan, and 5-FU) (n = 65) or seven 2-week cycles of modified FOLFIRINOX followed by stereotactic body radiotherapy (33 Gy–40 Gy in 5 fractions) or hypofractionated image-guided radiotherapy (25 Gy in 5 fractions) (n = 55). Patients without disease progression then underwent surgery followed by four 2-week cycles of adjuvant FOLFOX6 (oxaliplatin, leucovorin, and 5-FU).[
12 ]- In the neoadjuvant chemotherapy-alone arm, the median overall survival (OS) was 29.8 months (95% confidence interval [CI], 21.1–36.6) with a 43% microscopically margin-negative (R0) resection rate. This was compared with estimated historical controls of median OS at 18 months.[
12 ][Level of evidence C3] - Grade 3 or greater treatment-related adverse events occurred in 57% of patients who received neoadjuvant chemotherapy alone.
- The neoadjuvant chemotherapy with radiation arm was closed at interim futility analysis because of low R0 resection rates (33%) in the first 30 patients enrolled.
- In the neoadjuvant chemotherapy-alone arm, the median overall survival (OS) was 29.8 months (95% confidence interval [CI], 21.1–36.6) with a 43% microscopically margin-negative (R0) resection rate. This was compared with estimated historical controls of median OS at 18 months.[
- The multicenter phase III PREOPANC trial included 246 patients diagnosed with resectable or borderline resectable pancreatic cancer between 2013 and 2017. Patients at 16 Dutch centers were randomly assigned to receive either diagnostic laparoscopy, neoadjuvant chemoradiation therapy, surgical resection, and four cycles of adjuvant gemcitabine or up-front surgery and six cycles of adjuvant gemcitabine. Neoadjuvant chemoradiation therapy included the following: cycle 1 (21 days) with gemcitabine 1,000 mg/m2 on days 1 and 8; cycle 2 (28 days) with gemcitabine on days 1, 8, and 15 with 15 concurrent fractions of hypofractionated radiation (36 Gy) to the tumor and suspected associated lymph nodes; and cycle 3 (21 days) with gemcitabine on days 1 and 8.[
13 ]- The 5-year OS rate was 20.5% (95% CI, 14.2%–29.8%) for patients who received neoadjuvant chemoradiation therapy and 6.5% (95% CI, 3.1%–13.7%) for patients who received up-front surgery (hazard ratio [HR], 0.73; 95% CI, 0.56–0.96; P = .025).[
13 ][Level of evidence A1] - The median OS was 15.7 months in the neoadjuvant chemoradiation therapy group and 14.3 months in the up-front surgery group.
- In the intention-to-treat arm, 61% of patients who received neoadjuvant chemoradiation therapy underwent resection, resulting in R0 resection for 41% of patients and node-negative disease for 65% of patients. The resection rate was 72% in the up-front surgery arm, resulting in R0 resection in 28% of patients and node-negative disease in 18% of patients.
The optimal neoadjuvant therapy regimen is unknown, and additional chemotherapy regimens are being evaluated in the following trials: ALLIANCE (NCT04340141), PREOPANC-3 (NCT04927780), PANACHE-01-PRODIGE (NCT02959879), and NorPACT-01 (NCT02919787).
- The 5-year OS rate was 20.5% (95% CI, 14.2%–29.8%) for patients who received neoadjuvant chemoradiation therapy and 6.5% (95% CI, 3.1%–13.7%) for patients who received up-front surgery (hazard ratio [HR], 0.73; 95% CI, 0.56–0.96; P = .025).[
Surgery
Complete resection can yield 5-year survival rates of 18% to 24%, but ultimate control remains poor because of the high incidence of both local and distant tumor recurrence. Thus, systemic therapy is also recommended.[
Approximately 20% of patients present with pancreatic cancer amenable to local surgical resection, with operative mortality rates of approximately 1% to 16%.[
Postoperative chemotherapy
Historically, multiple randomized trials have established that adjuvant gemcitabine monotherapy [
For patients with good performance status, adjuvant FOLFIRINOX chemotherapy or the combination of gemcitabine and capecitabine should be considered. However, for older patients or patients with marginal performance status, adjuvant gemcitabine or 5-FU monotherapy can be considered. In Asia, S-1 (tegafur, gimeracil, and oteracil potassium) is an appropriate alternative to gemcitabine-based therapies.
Evidence (postoperative chemotherapy):
- FOLFIRINOX: In the randomized, open-label, phase III PRODIGE-24 trial (NCT01526135), 493 patients with R0/R1 resections were randomly assigned 1:1 to receive six cycles of gemcitabine (1,000 mg/m2 on days 1, 8, and 15 of a 28-day cycle) or 12 cycles of FOLFIRINOX (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 150 mg/m2, and 5-FU 2,400 mg/m2 over 46 hours every 2 weeks).[
23 ,24 ][Level of evidence A1]- With a median follow-up of 69.7 months, the median disease-free survival (DFS) was 21.4 months (95% CI, 17.5–26.7) in the FOLFIRINOX group and 12.8 months in the gemcitabine group (95% CI, 11.6–15.2) (HR, 0.66; 95% CI, 0.54–0.82; P < .001).
- The median OS was 53.5 months (95% CI, 43.5–58.4) in the FOLFIRINOX group and 35.5 months (95% CI, 30.1–40.3) in the gemcitabine group (HR, 0.68; 95% CI, 0.54–0.85; P = .001). The 5-year OS rate was 43.2% in the FOLFIRINOX group and 31.4% in the gemcitabine group.
- Toxicity was higher with combination therapy; 75.9% of patients who received FOLFIRINOX had grade 3 or 4 toxicities, compared with 52.9% of those who received gemcitabine, with similar rates of neutropenia (although 62.2% of patients on FOLFIRINOX received granulocyte colony-stimulating factor). Thirty-three percent of patients who received FOLFIRINOX stopped treatment prematurely, compared with 21% of patients who received gemcitabine alone.
- Gemcitabine and capecitabine: The European Study for Pancreatic Cancer (ESPAC-4 [NCT00058201]) trial randomly assigned 732 patients with resected pancreatic cancer to receive either six cycles of gemcitabine alone (1,000 mg/m2 given weekly for 3 weeks of every 4 weeks) or oral capecitabine (1,660 mg/m2 given for 21 days followed by 7 days of rest [one cycle]).[
25 ][Level of evidence A1]- With a median follow-up of 43.2 months, the median OS for patients in the gemcitabine/capecitabine group was 28.0 months (95% CI, 23.5–31.5) compared with 25.5 months for the gemcitabine-alone group (95% CI, 22.7–27.9; HR, 0.82; P = .032). Treatment with gemcitabine/capecitabine yielded an improvement in the estimated 5-year OS rate from 16.3% with gemcitabine alone to 28.8% with gemcitabine/capecitabine.
- There was no significant difference in overall rates of grade 3/4 toxicities between treatment arms. Compared with gemcitabine alone, capecitabine was associated with higher rates of grade 3/4 diarrhea (5% vs. 2%), neutropenia (38% vs. 24%), and hand-foot syndrome (7% vs. 0%).
- There was no significant effect on the quality of life in the treatment groups.
- Based on these findings, the adjuvant combination of gemcitabine and capecitabine is the standard of care after a resection for pancreatic cancer.
- S-1: The Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC-01) study was a phase III, multicenter, noninferiority trial conducted in Japan that randomly assigned 385 patients to receive either gemcitabine (1,000 mg/m2 weekly for 3 weeks of every 4 weeks) for six cycles or S-1 (tegafur, gimeracil, and oteracil potassium) (given orally twice a day for 4 weeks followed by a 2-week break).[
26 ][Level of evidence A1]- The prespecified criteria for early discontinuation was met at interim analysis for efficacy with all of the protocol treatments completed. On early interim analysis, the HRmortality was 0.57 (95% CI, 0.44–0.72; P for noninferiority < .001; P for superiority < .001). These results were associated with a 5-year OS rate of 24.4% in the gemcitabine group and 44.1% in the S-1 group.
- Grade 3 or 4 leukopenia, neutropenia, and liver transaminitis were observed more frequently in the gemcitabine group, and stomatitis and diarrhea were experienced more frequently in the S-1 group.
- Among Japanese patients, adjuvant chemotherapy with S-1 can be a new standard of care for resected pancreatic patients. Additional studies are needed to validate these results in patients of other races and ethnicities.
- The pharmacokinetics and pharmacodynamics of S-1 may be different between Eastern and Western patient populations because grade 3/4 gastrointestinal toxicities, especially diarrhea, have been reported more commonly in the Western patient population. S-1 is not currently approved by the U.S. Food and Drug Administration for use in the United States.
- Gemcitabine: Charité Onkologie (CONKO)-001 was a multicenter phase III trial of 368 patients with resected pancreatic cancer who were randomly assigned to receive six cycles of adjuvant gemcitabine versus observation.[
22 ][Level of evidence B1] In contrast to the previous trials, the primary end point was DFS.- With a median follow-up of 136 months, long-term follow-up of the CONKO-001 study demonstrated a significant improvement in OS that favors gemcitabine (median survival, 22.8 months vs. 20.2 months; HR, 0.76; 95% CI, 0.61–0.95, P = .01). Gemcitabine compared with observation alone yielded improved survival rates at 5 years of 20.7% for the gemcitabine arm versus 10.4% for the observation-alone arm, and the survival rates at 10 years were 12.2% for the gemcitabine arm versus 7.7% for the observation-alone arm.[
27 ][Level of evidence A1]
- With a median follow-up of 136 months, long-term follow-up of the CONKO-001 study demonstrated a significant improvement in OS that favors gemcitabine (median survival, 22.8 months vs. 20.2 months; HR, 0.76; 95% CI, 0.61–0.95, P = .01). Gemcitabine compared with observation alone yielded improved survival rates at 5 years of 20.7% for the gemcitabine arm versus 10.4% for the observation-alone arm, and the survival rates at 10 years were 12.2% for the gemcitabine arm versus 7.7% for the observation-alone arm.[
- Gemcitabine or 5-FU: The ESPAC-3 trial (NCT00058201) randomly assigned 1,088 patients who had undergone complete macroscopic resection to either 6 months of 5-FU (425 mg/m2) and leucovorin (20 mg/m2) on days 1 to 5 every 28 days or 6 months of gemcitabine (1,000 mg/m2) on days 1, 8, and 15 every 28 days.[
3 ][Level of evidence A1]- Median OS was 23.0 months (95% CI, 21.1–25.0) for patients treated with 5-FU plus leucovorin and 23.6 months (95% CI, 21.4–26.4) for those treated with gemcitabine (HR, 0.94; 95% CI, 0.81–1.08; P = .39).
Postoperative chemoradiation therapy
The role of postoperative therapy (chemotherapy with or without chemoradiation therapy) in the management of this disease remains controversial because much of the randomized clinical trial data available are statistically underpowered and provide conflicting results.[
Evidence (postoperative chemoradiation therapy):
Several phase III trials examined the potential OS benefit of postoperative adjuvant 5-FU–based chemoradiation therapy:
- Gastrointestinal Study Group (GITSG): A small randomized trial conducted by the
GITSG in 1985 compared surgery alone with surgery followed by chemoradiation.[4 ][Level of evidence A1];[5 ][Level of evidence B4]- The investigators reported a significant but modest improvement in median-term and long-term survival over resection alone with postoperative bolus 5-FU and regional split-course radiation given at a dose of 40 Gy.
- European Organisation for the Research and Treatment of Cancer (EORTC): An attempt by the EORTC to reproduce the results of the GITSG trial failed to confirm a significant benefit for adjuvant chemoradiation therapy over resection alone;[
6 ][Level of evidence A1] however, this trial treated patients with pancreatic and periampullary cancers (with a potentially better prognosis).- A subset analysis of the patients with primary pancreatic tumors indicated a trend toward improved median, 2-year, and 5-year OS with adjuvant therapy (17.1 months, 37%, and 20%, respectively) compared with surgery alone (12.6 months, 23%, and 10%, respectively); P = .09 for median survival).
- An updated analysis of a subsequent ESPAC-1 trial examined only patients who underwent strict randomization after pancreatic resection. The patients were assigned to one of four groups (observation, bolus 5-FU chemotherapy, bolus 5-FU chemoradiation therapy, or chemoradiation therapy followed by additional chemotherapy).[
7 ,8 ,28 ][Level of evidence A1]- With a 2 × 2 factorial design reported at a median follow-up of 47 months, a median survival benefit was observed for only the patients who received postoperative 5-FU chemotherapy. However, these results were difficult to interpret because of a high rate of protocol nonadherence and the lack of a separate analysis for each of the four groups in the 2 × 2 design.
- U.S. Gastrointestinal Intergroup: The U.S. Gastrointestinal Intergroup has reported the results of a randomized phase III trial (Radiation Therapy Oncology Group [RTOG]-9704) that included 451 patients with resected pancreatic cancers who were assigned to receive either postoperative infusional 5-FU plus infusional 5-FU and concurrent radiation or adjuvant gemcitabine plus infusional 5-FU and concurrent radiation.[
29 ][Level of evidence A1] The primary end points were OS for all patients and OS for patients with pancreatic head tumors.- A 5-year update of RTOG-9704 reported that patients with pancreatic head tumors (n = 388) had a median survival of 20.5 months and a 5-year OS rate of 22% with gemcitabine, versus a median survival of 17.1 months and a 5-year OS rate of 18% with 5-FU (HR, 0.84; 95% CI, 0.67–1.05; P = .12).[
30 ] - Univariate analysis showed no difference in OS. However, on multivariate analysis, patients on the gemcitabine arm with pancreatic head tumors experienced a trend toward improved OS (P = .08). Distant relapse remained the predominant site of first failure (78%).
- A 5-year update of RTOG-9704 reported that patients with pancreatic head tumors (n = 388) had a median survival of 20.5 months and a 5-year OS rate of 22% with gemcitabine, versus a median survival of 17.1 months and a 5-year OS rate of 18% with 5-FU (HR, 0.84; 95% CI, 0.67–1.05; P = .12).[
The EORTC/U.S. Gastrointestinal Intergroup RTOG-0848 phase III adjuvant trial evaluating the impact of chemoradiation therapy after completion of a full course of gemcitabine with or without erlotinib has closed and results are pending.
Additional trials are still warranted to determine more effective systemic therapy for this disease.
Current Clinical Trials
Use our
References:
- Dalton RR, Sarr MG, van Heerden JA, et al.: Carcinoma of the body and tail of the pancreas: is curative resection justified? Surgery 111 (5): 489-94, 1992.
- Brennan MF, Moccia RD, Klimstra D: Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg 223 (5): 506-11; discussion 511-2, 1996.
- Neoptolemos JP, Stocken DD, Bassi C, et al.: Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304 (10): 1073-81, 2010.
- Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer 59 (12): 2006-10, 1987.
- Kalser MH, Ellenberg SS: Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 120 (8): 899-903, 1985.
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al.: Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230 (6): 776-82; discussion 782-4, 1999.
- Neoptolemos JP, Dunn JA, Stocken DD, et al.: Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 358 (9293): 1576-85, 2001.
- Neoptolemos JP, Stocken DD, Friess H, et al.: A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350 (12): 1200-10, 2004.
- Stessin AM, Meyer JE, Sherr DL: Neoadjuvant radiation is associated with improved survival in patients with resectable pancreatic cancer: an analysis of data from the surveillance, epidemiology, and end results (SEER) registry. Int J Radiat Oncol Biol Phys 72 (4): 1128-33, 2008.
- Versteijne E, Vogel JA, Besselink MG, et al.: Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 105 (8): 946-958, 2018.
- Mokdad AA, Minter RM, Zhu H, et al.: Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol 35 (5): 515-522, 2017.
- Katz MHG, Shi Q, Meyers J, et al.: Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol 8 (9): 1263-1270, 2022.
- Versteijne E, van Dam JL, Suker M, et al.: Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol 40 (11): 1220-1230, 2022.
- Cameron JL, Crist DW, Sitzmann JV, et al.: Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg 161 (1): 120-4; discussion 124-5, 1991.
- Yeo CJ, Cameron JL, Lillemoe KD, et al.: Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 221 (6): 721-31; discussion 731-3, 1995.
- Yeo CJ, Abrams RA, Grochow LB, et al.: Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 225 (5): 621-33; discussion 633-6, 1997.
- Birkmeyer JD, Finlayson SR, Tosteson AN, et al.: Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 125 (3): 250-6, 1999.
- Cameron JL, Pitt HA, Yeo CJ, et al.: One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 217 (5): 430-5; discussion 435-8, 1993.
- Spanknebel K, Conlon KC: Advances in the surgical management of pancreatic cancer. Cancer J 7 (4): 312-23, 2001 Jul-Aug.
- Balcom JH, Rattner DW, Warshaw AL, et al.: Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg 136 (4): 391-8, 2001.
- Sohn TA, Yeo CJ, Cameron JL, et al.: Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 4 (6): 567-79, 2000 Nov-Dec.
- Oettle H, Post S, Neuhaus P, et al.: Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297 (3): 267-77, 2007.
- Conroy T, Hammel P, Hebbar M, et al.: FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 379 (25): 2395-2406, 2018.
- Conroy T, Castan F, Lopez A, et al.: Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol 8 (11): 1571-1578, 2022.
- Neoptolemos JP, Palmer DH, Ghaneh P, et al.: Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389 (10073): 1011-1024, 2017.
- Uesaka K, Boku N, Fukutomi A, et al.: Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388 (10041): 248-57, 2016.
- Oettle H, Neuhaus P, Hochhaus A, et al.: Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310 (14): 1473-81, 2013.
- Choti MA: Adjuvant therapy for pancreatic cancer--the debate continues. N Engl J Med 350 (12): 1249-51, 2004.
- Regine WF, Winter KA, Abrams RA, et al.: Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 299 (9): 1019-26, 2008.
- Regine WF, Winter KA, Abrams R, et al.: Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol 18 (5): 1319-26, 2011.
Treatment of Locally Advanced Pancreatic Cancer
Treatment Options for Locally Advanced Pancreatic Cancer
While locally advanced and metastatic pancreatic cancer are both incurable, their natural histories may be different. An autopsy series demonstrated that 30% of patients presenting with locally advanced disease died without evidence of distant metastases.[
Treatment options for locally advanced pancreatic cancer include:
-
Chemotherapy with or without targeted therapy . -
Chemoradiation therapy : Chemotherapy followed by chemoradiation for patients without metastatic disease. -
Surgery : Radical pancreatic resection. -
Palliative surgery : Palliative surgical biliary and/or gastric bypass, percutaneous radiologic biliary stent placement, or endoscopic biliary stent placement.[2 ,3 ] - Clinical trials evaluating novel agents in combination with chemotherapy or chemoradiation therapy for patients with unresectable tumors.
- Intraoperative radiation therapy and/or implantation of radioactive sources (under clinical evaluation).[
4 ,5 ]
Palliative therapies can be considered in patients with any stage of disease. For more information, see the
Chemotherapy with or without targeted therapy
Chemotherapy is the primary treatment modality for patients with locally advanced pancreatic cancers and uses the same regimens as those used to treat patients with metastatic disease.
Evidence (chemotherapy):
- FOLFIRINOX versus gemcitabine: A multicenter phase II/III trial included 342 patients with metastatic pancreatic adenocarcinoma with an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1.[
6 ][Level of evidence A1] The patients were randomly assigned to receive FOLFIRINOX (oxaliplatin [85 mg/m2], irinotecan [180 mg/m2], leucovorin [400 mg/m2], and fluorouracil [5-FU; 400 mg/m2] given as a bolus followed by 2,400 mg/m2 given as a 46-hour continuous infusion, every 2 weeks) or gemcitabine (1,000 mg/m2 weekly for 7 of 8 weeks and then weekly for 3 of 4 weeks).- The median overall survival (OS) was 11.1 months in the FOLFIRINOX group compared with 6.8 months in the gemcitabine group (hazard ratio [HR]death, 0.57; 95% confidence interval [CI], 0.45–0.73; P < .001).
- Median progression-free survival (PFS) was 6.4 months in the FOLFIRINOX group and 3.3 months in the gemcitabine group (HRdisease progression, 0.47; 95% CI, 0.37–0.59; P < .001).
- FOLFIRINOX was more toxic than gemcitabine; 5.4% of patients in this group had febrile neutropenia. At 6 months, 31% of the patients in the FOLFIRINOX group had a definitive degradation of quality of life, versus 66% in the gemcitabine group (HR, 0.47; 95% CI, 0.30–0.70; P < .001).
- Based on this trial, FOLFIRINOX is considered a standard treatment option for patients with advanced pancreatic cancer.
- Gemcitabine and nab-paclitaxel versus gemcitabine: A multicenter, international, phase III trial (NCT00844649) included 861 patients with metastatic pancreatic adenocarcinoma. Patients had a Karnofsky Performance Status of at least 70 and had not previously received chemotherapy for metastatic disease.[
7 ][Level of evidence A1] Patients who received adjuvant gemcitabine or any other chemotherapy were excluded. The patients were randomly assigned to receive gemcitabine (1,000 mg/m2) and nab-paclitaxel (125 mg/m2 of body-surface area) weekly for 3 of 4 weeks or gemcitabine monotherapy (1,000 mg/m2 weekly for 7 of 8 weeks and then weekly for 3 of 4 weeks).- The median OS was 8.5 months in the nab-paclitaxel/gemcitabine group compared with 6.7 months in the gemcitabine group (HRdeath, 0.72; 95% CI, 0.62–0.83; P < .001).
- Median PFS was 5.5 months in the nab-paclitaxel/gemcitabine group and 3.7 months in the gemcitabine group (HRdisease progression, 0.69; 95% CI, 0.58–0.82; P < .001).
- Nab-paclitaxel/gemcitabine was more toxic than gemcitabine. The most common grade 3 toxicities in the nab-paclitaxel/gemcitabine group were neutropenia (38%), fatigue (17%), and neuropathy (17%); febrile neutropenia occurred in 3% of patients. In the gemcitabine-alone group, the most common grade 3 toxicities were neutropenia (27%), fatigue (1%), and neuropathy (1%); febrile neutropenia occurred in 1% of patients.
- In the nab-paclitaxel/gemcitabine group, the median time from grade 3 neuropathy to grade 1 or resolution was 29 days. Of patients with grade 3 peripheral neuropathy, 44% were able to resume treatment at a reduced dose within a median of 23 days after onset of a grade 3 event.
- Based on this trial, nab-paclitaxel/gemcitabine is a standard treatment option for patients with advanced pancreatic cancer.
- Quality-of-life data were not measured for this regimen, and this study did not address the efficacy of nab-paclitaxel/gemcitabine versus FOLFIRINOX.
- Gemcitabine versus 5-FU: Gemcitabine has demonstrated activity in patients with pancreatic cancer and is a useful palliative agent.[
8 ,9 ,10 ] A phase III trial of gemcitabine versus 5-FU as first-line therapy in patients with advanced or metastatic adenocarcinoma of the pancreas reported a significant improvement in survival among patients treated with gemcitabine (the 1-year survival rate was 18% with gemcitabine compared with 2% with 5-FU; P = .003).[9 ][Level of evidence A1] - Gemcitabine alone versus gemcitabine and erlotinib: The National Cancer Institute of Canada performed a phase III trial (CAN-NCIC-PA3 [NCT00026338]) that compared gemcitabine alone with the combination of gemcitabine and erlotinib (100 mg/d) in patients with advanced or metastatic pancreatic carcinomas.[
11 ][Level of evidence A1]- The addition of erlotinib modestly prolonged survival when combined with gemcitabine versus gemcitabine alone (HR, 0.81; 95% CI, 0.69–0.99; P = .038).
- The median and 1-year survival rates for patients who received erlotinib were 6.2 months and 23%. The median and 1-year survival rates for patients who received placebo were 5.9 months and 17%.
- Platinum analogue or fluoropyrimidine versus single-agent gemcitabine: Many phase III studies have evaluated a combination regimen with either a platinum analogue (cisplatin or oxaliplatin) or fluoropyrimidine versus single-agent gemcitabine.[
12 ,13 ]- None of these phase III trials have demonstrated a statistically significant advantage favoring the use of combination chemotherapy in the first-line treatment of metastatic pancreatic cancer.
- 5-FU, leucovorin, and oxaliplatin (OFF regimen) versus best supportive care (BSC): Second-line chemotherapy after progression on a gemcitabine-based regimen may be beneficial. The Charité Onkologie (CONKO)
-003 investigators randomly assigned patients requiring a second line of chemotherapy to either the OFF regimen or BSC.[14 ,15 ][Level of evidence C1] The OFF regimen consisted of leucovorin (200 mg/m2) followed by 5-FU (2,000 mg/m2 [24-hour continuous infusion] on days 1, 8, 15, and 22) and oxaliplatin (85 mg/m2 on days 8 and 22). After a rest of 3 weeks, the next cycle was started on day 43. The trial was terminated early because of poor accrual, and only 46 patients were randomly assigned to either the OFF regimen or BSC.- The median survival was 4.82 months (95% CI, 4.29–5.35) with the OFF treatment regimen and 2.30 months (95% CI, 1.76–2.83) with BSC alone (HR, 0.45; 95% CI, 0.24–0.83).
- Median OS was 9.09 months for the sequence of gemcitabine/OFF and 7.90 months for gemcitabine/BSC.
- The early closure of the study and the very small number of patients made the P values misleading. Therefore, second-line chemotherapy with the OFF regimen may be falsely associated with improved survival.
Chemoradiation therapy
The role of chemoradiation in locally advanced pancreatic cancer remains controversial.
| Trial | Regimen | Chemoradiation | Radiation Alone | Chemotherapy Alone | P Value |
|---|---|---|---|---|---|
| 5-FU = fluorouracil; ECOG = Eastern Cooperative Oncology Group; FFCD = Fédération Francophone de Cancérologie Digestive; GEM = gemcitabine; GITSG = Gastrointestinal Tumor Study Group; Gy = gray (unit of absorbed radiation of ionizing radiation);P value = probability value; XRT = radiation therapy. | |||||
| Pre-2000 | |||||
| GITSG[ |
Radiation alone vs. 5-FU/60 Gy XRT | 40 wk | 20 wk | <.01 | |
| ECOG[ |
Radiation vs. 5-FU, mitomycin C/59 Gy XRT | 8.4 mo | 7.1 mo | .16 | |
| Post-2000 | |||||
| FFCD[ |
GEM vs. GEM, cisplatin, 60 Gy XRT | 8.6 mo | 13 mo | .03 | |
| ECOG[ |
GEM vs. GEM/50.4 Gy XRT | 11.1 mo | 9.2 mo | .017 | |
Evidence (chemoradiation therapy):
Three trials evaluated combined modality therapy versus radiation therapy alone.[
- LAP07 (NCT00634725): The LAP07 study was an international, randomized, phase III study based on the results of the Groupe Coopérateur Multidisciplinaire en Oncologie (GERCOR) study. In total, 449 patients were enrolled between 2008 and 2011, with random assignment via a two-step randomization process. In the first step, patients were randomly assigned to induction gemcitabine (n = 223) or gemcitabine plus erlotinib (n = 219) for four cycles. For the second step, patients with controlled tumors were randomly assigned (n = 269) a second time to receive either chemotherapy (n = 136) or chemoradiation therapy (n = 133). A total dose of 54 Gy in 30 daily fractions was prescribed with concurrent capecitabine at a dose of 800 mg/m2 twice daily on days of radiation therapy.[
20 ][Level of evidence A1]- The primary end point was OS. After interim analysis, the study was stopped early because of futility.
- With a median follow-up of 36.7 months, the median OS from the date of the first randomization was not significantly different between chemotherapy at 16.5 months (95% CI, 14.5–18.5) and chemoradiation therapy at 15.2 months (95% CI, 13.9–17.3; P = .83).
- Median OS after the first randomization was 13.6 months (95% CI, 12.3–15.3) for the patients who received gemcitabine and was 11.9 months (95% CI, 10.4–13.5; P = .09) for the patients who received gemcitabine plus erlotinib.
The LAP07 study represents the most robust, prospective, randomized phase III data regarding the role of chemoradiation therapy in the setting of gemcitabine-based induction chemotherapy that demonstrates no OS benefit. However, this study was initiated before the advent of FOLFIRINOX chemotherapy, which has been widely adopted into the locally advanced setting. The role of chemoradiation in the setting of more active chemotherapy regimens, including gemcitabine/paclitaxel and FOLFIRINOX, has yet to be evaluated.
- Gastrointestinal Tumor Study Group (GITSG) GITSG-9273 trial: Before 2000, several phase III trials evaluated combined modality therapy versus radiation therapy alone. Before the use of gemcitabine for patients with locally advanced or metastatic pancreatic cancer, investigators from the GITSG randomly assigned 106 patients with locally advanced pancreatic adenocarcinoma to receive external-beam radiation therapy (EBRT) (60 Gy) alone or concurrent EBRT (either 40 Gy or 60 Gy) plus bolus 5-FU.[
16 ][Level of evidence A1]- The study was stopped early when the chemoradiation therapy groups were found to have better efficacy. The 1-year survival rate was 11% for patients who received EBRT alone compared with 38% for patients who received chemoradiation therapy with 40 Gy and 36% for patients who received chemoradiation therapy with 60 Gy.
- After an additional 88 patients were enrolled in the combined modality arms, there was a trend toward improved survival with 60 Gy EBRT plus 5-FU, but the difference in time-to-progression and OS was not statistically significant when compared with the 40 Gy arm.[
21 ]
- ECOG E-8282 trial: Investigators from the ECOG randomly assigned 114 patients to receive radiation therapy (59.4 Gy) alone or with concurrent infusional 5-FU (1,000 mg/m2 /d on days 2–5 and 28–31) plus mitomycin (10 mg/m2 on day 2).[
17 ]- The trial reported no difference in OS between the two groups.
- Fédération Francophone de Cancérologie Digestive–Société Française de Radiothérapie Oncologie (FFCD-SFRO) trial: As it became clear that radiation therapy alone was an inadequate treatment, investigators evaluated combined modality approaches versus chemotherapy alone. Investigators from the FFCD-SFRO randomly assigned 119 patients to induction chemoradiation therapy (60 Gy in 2 Gy fractions with 300 mg/m2 /d of continuous-infusion 5-FU on days 1–5 for 6 weeks and 20 mg/m2 /d of cisplatin on days 1–5 during weeks 1 and 5) or induction gemcitabine (1,000 mg/m2 weekly for 7 weeks). Maintenance gemcitabine was administered to both groups until stopped by disease progression or treatment discontinuation as a result of toxicity.[
22 ][Level of evidence A1]- Median survival was superior in the gemcitabine group (13 vs. 8.6 months; P = .03).
- Nonhematological grade 3 to 4 toxicities (primarily gastrointestinal) were significantly more common in the chemoradiation therapy group (44% vs. 18%; P = .004), and fewer patients completed at least 75% of induction therapy (42% vs. 73%).
- Nonetheless, the survival benefit persisted in a per-protocol analysis of patients receiving at least 75% of planned therapy. Notably, the dose intensity of maintenance gemcitabine was significantly less in the chemoradiation therapy group because of a greater incidence of grades 3 to 4 hematological toxicities (71% vs. 27%; P = .0001).
- As a result of this study, giving induction chemoradiation therapy has lost support.
- ECOG: The results of the FFCD-SFRO study counter the results of a study from ECOG in which investigators randomly assigned 74 patients to either gemcitabine alone or gemcitabine with radiation followed by gemcitabine.[
19 ] Of note, the study was closed early as the result of poor accrual.- The primary end point was survival, which was 9.2 months (95% CI, 7.9–11.4) for chemotherapy and 11.1 months (95% CI, 7.6–15.5) for combined modality therapy (one-sided P = .017 by stratified log-rank test).
- Grades 4 and 5 toxicity were greater in the chemoradiation therapy arm than in the chemotherapy arm (41% vs. 9%).
- GERCOR: Given the increased toxicity of chemoradiation therapy and the early development of metastatic disease in a large percentage of patients with locally advanced pancreatic cancer, investigators are pursuing a strategy of selecting patients with localized disease for chemoradiation therapy. With this strategy, the selected patients have an absence of progressive disease locally or systemically after several months of chemotherapy.[
23 ][Level of evidence C1]- A retrospective analysis of 181 patients enrolled in prospective phase II and III GERCOR studies revealed that 29% had metastatic disease after 3 months of gemcitabine-based chemotherapy.
- For the remaining 71%, median OS was significantly longer among patients treated with chemoradiation therapy than among patients treated with additional chemotherapy (15.0 months vs. 11.7 months; P = .0009).
Surgery
Patients with locally advanced pancreatic cancer have tumors that are technically unresectable because of local vessel impingement or invasion by tumor. However, with the combination of chemotherapy and chemoradiation therapy, some patients may become candidates for radical pancreatic resection.
Palliative surgery
A significant proportion (approximately one-third) of patients with pancreatic cancer present with locally advanced disease. Patients may benefit from palliation of biliary obstruction by endoscopic, surgical, or radiological means.[
Current Clinical Trials
Use our
References:
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al.: DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 27 (11): 1806-13, 2009.
- van den Bosch RP, van der Schelling GP, Klinkenbijl JH, et al.: Guidelines for the application of surgery and endoprostheses in the palliation of obstructive jaundice in advanced cancer of the pancreas. Ann Surg 219 (1): 18-24, 1994.
- Baron TH: Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 344 (22): 1681-7, 2001.
- Tepper JE, Noyes D, Krall JM, et al.: Intraoperative radiation therapy of pancreatic carcinoma: a report of RTOG-8505. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 21 (5): 1145-9, 1991.
- Reni M, Panucci MG, Ferreri AJ, et al.: Effect on local control and survival of electron beam intraoperative irradiation for resectable pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 50 (3): 651-8, 2001.
- Conroy T, Desseigne F, Ychou M, et al.: FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364 (19): 1817-25, 2011.
- Von Hoff DD, Ervin T, Arena FP, et al.: Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369 (18): 1691-703, 2013.
- Rothenberg ML, Moore MJ, Cripps MC, et al.: A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol 7 (4): 347-53, 1996.
- Burris HA, Moore MJ, Andersen J, et al.: Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15 (6): 2403-13, 1997.
- Storniolo AM, Enas NH, Brown CA, et al.: An investigational new drug treatment program for patients with gemcitabine: results for over 3000 patients with pancreatic carcinoma. Cancer 85 (6): 1261-8, 1999.
- Moore MJ, Goldstein D, Hamm J, et al.: Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25 (15): 1960-6, 2007.
- Poplin E, Feng Y, Berlin J, et al.: Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 27 (23): 3778-85, 2009.
- Colucci G, Labianca R, Di Costanzo F, et al.: Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 28 (10): 1645-51, 2010.
- Pelzer U, Kubica K, Stieler J, et al.: A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. [Abstract] J Clin Oncol 26 (Suppl 15): A-4508, 2008.
- Pelzer U, Schwaner I, Stieler J, et al.: Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 47 (11): 1676-81, 2011.
- A multi-institutional comparative trial of radiation therapy alone and in combination with 5-fluorouracil for locally unresectable pancreatic carcinoma. The Gastrointestinal Tumor Study Group. Ann Surg 189 (2): 205-8, 1979.
- Cohen SJ, Dobelbower R, Lipsitz S, et al.: A randomized phase III study of radiotherapy alone or with 5-fluorouracil and mitomycin-C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group study E8282. Int J Radiat Oncol Biol Phys 62 (5): 1345-50, 2005.
- Chauffert B, Mornex F, Bonnetain F, et al.: Phase III trial comparing initial chemoradiotherapy (intermittent cisplatin and infusional 5-FU) followed by gemcitabine vs. gemcitabine alone in patients with locally advanced non metastatic pancreatic cancer: a FFCD-SFRO study. [Abstract] J Clin Oncol 24 (Suppl 18): A-4008, 180s, 2006.
- Loehrer PJ, Feng Y, Cardenes H, et al.: Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 29 (31): 4105-12, 2011.
- Hammel P, Huguet F, van Laethem JL, et al.: Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 315 (17): 1844-53, 2016.
- Moertel CG, Frytak S, Hahn RG, et al.: Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 48 (8): 1705-10, 1981.
- Chauffert B, Mornex F, Bonnetain F, et al.: Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 19 (9): 1592-9, 2008.
- Huguet F, André T, Hammel P, et al.: Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 25 (3): 326-31, 2007.
- Sohn TA, Lillemoe KD, Cameron JL, et al.: Surgical palliation of unresectable periampullary adenocarcinoma in the 1990s. J Am Coll Surg 188 (6): 658-66; discussion 666-9, 1999.
Treatment of Metastatic or Recurrent Pancreatic Cancer
Treatment Options for Metastatic or Recurrent Pancreatic Cancer
Treatment options for metastatic or recurrent pancreatic cancer include:
-
Chemotherapy with or without targeted therapy . - Clinical trials evaluating new anticancer agents alone or in combination with chemotherapy.
Palliative therapies can be considered in patients with any stage of disease. For more information, see the
Chemotherapy with or without targeted therapy
Because of the low objective response rate and limited efficacy of palliative chemotherapy regimens, all newly diagnosed patients should consider enrolling in clinical trials. Multiagent chemotherapy combinations have been shown to prolong outcomes compared with single-agent gemcitabine.[
Evidence (single-agent chemotherapy):
- Gemcitabine versus fluorouracil (5-FU): A phase III trial of gemcitabine versus 5-FU as first-line therapy in patients with advanced or metastatic adenocarcinoma of the pancreas reported a significant improvement in survival among patients treated with gemcitabine (the 1-year survival rate was 18% with gemcitabine vs. 2% with 5-FU; P = .003).[
1 ][Level of evidence A1]
Evidence (multiagent chemotherapy):
- FOLFIRINOX (leucovorin, 5-FU, irinotecan, and oxaliplatin) versus gemcitabine: A multicenter phase II/III trial included 342 patients with metastatic pancreatic adenocarcinoma with an Eastern Cooperative Oncology Group performance status score of 0 or 1.[
4 ][Level of evidence A1] The patients were randomly assigned to receive FOLFIRINOX (oxaliplatin [85 mg/m2], irinotecan [180 mg/m2], leucovorin [400 mg/m2], and 5-FU [400 mg/m2] given as a bolus followed by 2,400 mg/m2 given as a 46-hour continuous infusion, every 2 weeks) or gemcitabine (1,000 mg/m2 weekly for 7 of 8 weeks and then weekly for 3 of 4 weeks).- The median overall survival (OS) was 11.1 months in the FOLFIRINOX group compared with 6.8 months in the gemcitabine group (hazard ratio [HR]death, 0.57; 95% confidence interval [CI], 0.45–0.73; P < .001).
- Median progression-free survival (PFS) was 6.4 months in the FOLFIRINOX group and 3.3 months in the gemcitabine group (HR for disease progression, 0.47; 95% CI, 0.37–0.59; P < .001).
- FOLFIRINOX was more toxic than gemcitabine; 5.4% of patients in this group had febrile neutropenia. At 6 months, 31% of the patients in the FOLFIRINOX group had a definitive degradation of quality of life versus 66% in the gemcitabine group (HR, 0.47; 95% CI, 0.30–0.70; P < .001).
- Based on this trial, FOLFIRINOX is considered a standard treatment option for patients with advanced pancreatic cancer.
- NALIRIFOX (5-FU, irinotecan sucrosofate [also called nanoliposomal irinotecan], and oxaliplatin) versus gemcitabine and nab-paclitaxel: The multicenter, open-label, phase III NAPOLI 3 study (NCT04083235) included 770 patients with confirmed pancreatic ductal adenocarcinoma who had not been treated previously for metastatic disease. The patients were randomly assigned 1:1 to receive NALIRIFOX (irinotecan sucrosofate [50 mg/m2], oxaliplatin [60 mg/m2], leucovorin [400 mg/m2], and 5-FU [2,400 mg/m2] as an intravenous infusion over 46 hours on days 1 and 15 of cycles lasting 28 days) or nab-paclitaxel (125 mg/m2) and gemcitabine (1,000 mg/m2 given intravenously on days 1, 8, and 15 of cycles lasting 28 days). The primary end point was OS from randomization to death due to any cause, for NALIRIFOX versus gemcitabine/nab-paclitaxel. Secondary end points included PFS and overall response rate by RECIST version 1.1.[
5 ]- The median OS was 11.1 months (95% CI, 10.0–12.1) in the NALIRIFOX group and 9.2 months (95% CI, 8.3–10.6) in the nab-paclitaxel/gemcitabine group (HR, 0.83; 95% CI, 0.7–0.99; P = .036). The median follow-up was 16.1 months.[
5 ][Level of evidence A1] - The median PFS was 7.4 months in the NALIRIFOX group and 5.6 months in the nab-paclitaxel/gemcitabine group (HR, 0.69; 95% CI, 0.58–0.83; P < .0001).
- The overall response rate was 42% in the NALIRIFOX group and 36% in the nab-paclitaxel/gemcitabine group (P = .11). The median duration of response was 7.3 months in the NALIRIFOX group and 5 months in the nab-paclitaxel/gemcitabine group (HR, 0.67; 95% CI, 0.48–0.93).
- There were 369 patients (>99%) with any adverse event in the NALIRIFOX group and 376 patients (99%) with any adverse event in the nab-paclitaxel/gemcitabine group. The most common grade 3 to 4 toxicities in the NALIRIFOX group were diarrhea (20%), hypokalemia (15%), neutropenia (14%), and nausea (12%). The most common grade 3 to 4 toxicities in the nab-paclitaxel/gemcitabine group were neutropenia (25%) and anemia (17%). Of note, hematological toxicities (i.e., neutropenia, anemia, thrombocytopenia) were lower in the NALIRIFOX group than the nab-paclitaxel/gemcitabine group. Peripheral neuropathy was noted in 3% of patients in the NALIRIFOX group compared with 6% of patients in the gemcitabine/nab-paclitaxel group.
Based on this trial, NALIRIFOX is a standard first-line treatment option for patients with advanced pancreatic cancer.
- The median OS was 11.1 months (95% CI, 10.0–12.1) in the NALIRIFOX group and 9.2 months (95% CI, 8.3–10.6) in the nab-paclitaxel/gemcitabine group (HR, 0.83; 95% CI, 0.7–0.99; P = .036). The median follow-up was 16.1 months.[
- Gemcitabine and nab-paclitaxel versus gemcitabine: A multicenter, international, phase III trial (NCT00844649) included 861 patients with metastatic pancreatic adenocarcinoma. Patients had a Karnofsky Performance Status of at least 70 and had not previously received chemotherapy for metastatic disease.[
6 ][Level of evidence A1] Patients who received adjuvant gemcitabine or any other chemotherapy were excluded. The patients were randomly assigned to receive gemcitabine (1,000 mg/m2) and nab-paclitaxel (125 mg/m2 of body-surface area) weekly for 3 of 4 weeks or gemcitabine monotherapy (1,000 mg/m2 weekly for 7 of 8 weeks and then weekly for 3 of 4 weeks).- The median OS was 8.5 months in the nab-paclitaxel/gemcitabine group compared with 6.7 months in the gemcitabine group (HRdeath, 0.72; 95% CI, 0.62–0.83; P < .001).
- Median PFS was 5.5 months in the nab-paclitaxel/gemcitabine group and 3.7 months in the gemcitabine group (HRdisease progression, 0.69; 95% CI, 0.58–0.82; P < .001).
- Nab-paclitaxel/gemcitabine was more toxic than gemcitabine. The most common grade 3 toxicities in the nab-paclitaxel/gemcitabine group were neutropenia (38%), fatigue (17%), and neuropathy (17%); febrile neutropenia occurred in 3% of patients. In the gemcitabine-alone group, the most common grade 3 toxicities were neutropenia (27%), fatigue (1%), and neuropathy (1%); febrile neutropenia occurred in 1% of patients.
- In the nab-paclitaxel/gemcitabine group, the median time from grade 3 neuropathy to grade 1 neuropathy or resolution was 29 days. Of patients with grade 3 peripheral neuropathy, 44% were able to resume treatment at a reduced dose within a median of 23 days after onset of a grade 3 event.
- Based on this trial, nab-paclitaxel plus gemcitabine is a standard treatment option for patients with advanced pancreatic cancer.
- Quality-of-life data were not measured for this regimen, and this study did not address the efficacy of nab-paclitaxel/gemcitabine versus FOLFIRINOX.
- Gemcitabine alone versus gemcitabine and erlotinib: The National Cancer Institute of Canada performed a phase III trial (CAN-NCIC-PA3 [NCT00026338]) that compared gemcitabine alone with the combination of gemcitabine and erlotinib (100 mg/d) in patients with advanced or metastatic pancreatic carcinomas.[
7 ][Level of evidence A1]- The addition of erlotinib modestly prolonged survival when combined with gemcitabine alone (HR, 0.81; 95% CI, 0.69–0.99; P = .038).
- The corresponding median survival rate for patients receiving erlotinib was 6.2 months versus 5.9 months for patients receiving placebo. The 1-year survival rate for patients receiving erlotinib was 23% versus 17% for patients receiving placebo.
Evidence (second-line chemotherapy):
- Irinotecan sucrosofate with or without 5-FU and leucovorin: The NAPOLI-1 trial (NCT01494506) evaluated the role of irinotecan sucrosofate in patients with metastatic pancreatic cancer who were previously treated with gemcitabine-based therapies.[
8 ] Irinotecan sucrosofate is an encapsulated formulation of irinotecan designed to increase intratumoral levels of irinotecan and its active metabolite. In this study, a total of 417 patients were randomly assigned to receive either irinotecan sucrosofate monotherapy (120 mg/m2 every 3 weeks; n = 151), 5-FU and leucovorin (n = 149), or irinotecan sucrosofate (80 mg/m2 every 2 weeks plus 5-FU) and leucovorin (n = 117).[8 ][Level of evidence B1]- Median OS was 6.1 months (95% CI, 4.8–8.9) in patients who received irinotecan sucrosofate with 5-FU and 4.2 months (95% CI, 3.6–4.9) in patients who received 5-FU and leucovorin (P = .012). Median OS was 4.9 months (95% CI, 4.2–5.6) for patients who received irinotecan sucrosofate monotherapy, compared with 4.2 months (95% CI, 3.6–4.9) for those who received 5-FU and leucovorin (unstratified HR, 0.99; P = .94). On multivariate analysis, irinotecan sucrosofate plus 5-FU and leucovorin was associated with improved OS (HR, 0.58; 95% CI, 0.42–0.81).
- Grade 3 or 4 adverse events occurred most frequently in the patients who received irinotecan sucrosofate plus 5-FU and leucovorin and included neutropenia (27%), diarrhea (13%), vomiting (11%), and fatigue (14%).
- Despite differences in survival and toxicity between regimens, quality of life was not significantly different between treatment groups.
- The benefit of using irinotecan sucrosofate rather than unencapsulated irinotecan has not been established because the regimen for the control arm of this study was 5-FU/leucovorin. Additionally, the value of using irinotecan sucrosofate after FOLFIRINOX in the first-line setting is not clear.
- 5-FU, leucovorin, and oxaliplatin (OFF regimen) versus best supportive care (BSC): Second-line chemotherapy after progression on a gemcitabine-based regimen may be beneficial. The Charité Onkologie (CONKO)-003 investigators randomly assigned patients requiring a second line of chemotherapy to either an OFF regimen or BSC.[
2 ]; [3 ][Level of evidence C1] The OFF regimen consisted of leucovorin (200 mg/m2) followed by 5-FU (2,000 mg/m2 [24 hours continuous infusion] on days 1, 8, 15, and 22) and oxaliplatin (85 mg/m2 on days 8 and 22). After a rest of 3 weeks, the next cycle was started on day 43. The trial was terminated early because of poor accrual, and only 46 patients were randomly assigned to either the OFF regimen or BSC.- The median survival was 4.82 months (95% CI, 4.29–5.35) with the OFF treatment regimen and 2.30 months (95% CI, 1.76–2.83) with BSC alone (HR, 0.45; 95% CI, 0.24–0.83).
- Median OS was 9.09 months for the sequence of gemcitabine/OFF and 7.90 months for gemcitabine/BSC.
- The early closure of the study and the very small number of patients made the P values misleading. Therefore, second-line chemotherapy with the OFF regimen may be falsely associated with improved survival.
- FOLFOX (leucovorin, 5-FU, and oxaliplatin) versus 5-FU/leucovorin after gemcitabine chemotherapy: The prospective, multicenter PANCREOX trial included 108 patients with advanced pancreatic cancer who had previously received first-line gemcitabine-based chemotherapy. Patients were randomly assigned to receive 5-FU/leucovorin without oxaliplatin (n = 54) or with oxaliplatin (n = 54), administered as modified FOLFOX-6 (mFOLFOX-6).[
9 ][Level of evidence C1] With a target accrual of 128 patients, the study closed prematurely because of slow accrual.- After a median follow-up of 8.8 months, the median PFS was 3.1 months in the mFOLFOX-6 arm and 2.9 months in the infusional 5-FU arm (HR, 1.00; 95% CI, 0.66–1.53, P = .989).
- Overall response rate and quality of life was not significantly different in the two arms.
- The overall incidence of grade 3 or 4 adverse events was 63% in the mFOLFOX-6 arm and 11% in the 5-FU/leucovorin arm. However, more patients in the mFOLFOX-6 arm withdrew from the study because of adverse events than did patients in the 5-FU/leucovorin arm (20% vs. 2%).
- Based on this study, no benefit was seen with the addition of oxaliplatin, administered in the mFOLFOX-6 regimen, versus infusional 5-FU/leucovorin among patients with advanced pancreatic cancer after first-line gemcitabine-based chemotherapy. These results may suggest that oxaliplatin-based regimens for metastatic pancreatic cancer may yield the greatest benefit in the first-line setting.
- Gemcitabine/paclitaxel versus gemcitabine after FOLFIRINOX failure or intolerance: The prospective, open-label, phase III GEMPAX study (NCT03943667) included 211 patients with metastatic pancreatic cancer that progressed during or within 3 months of completing first-line FOLFIRINOX (including FOLFIRINOX treatment as adjuvant therapy) or were intolerant of this therapy. Patients were randomly assigned to one of the following treatment arms:[
10 ]- Gemcitabine/paclitaxel: Gemcitabine (1,000 mg/m2) and solvent-based paclitaxel (80 mg/m2) on days 1, 8, and 15 of a 28-day cycle.
- Gemcitabine alone: Gemcitabine (1,000 mg/m2) on days 1, 8, and 15 of a 29-day cycle.
The following results were observed:
- There was no significant difference in OS among patients who received gemcitabine/paclitaxel (6.4 months after a median follow-up of 13.4 months) or gemcitabine alone (5.9 months after 13.8 months of follow-up) (HR, 0.87; 95% CI, 0.63–1.20; P = .4095). However, OS was improved in the following subgroups of patients who received gemcitabine/paclitaxel: (1) patients aged 65 years or younger (HR, 0.66; 95% CI, 0.44–0.99), and (2) patients with CA 19-9 levels of 59 times the upper limit of normal or higher at baseline (HR, 0.64; 95% CI, 0.42–0.97).[
10 ][Level of evidence B1] - PFS was 3.1 months in the gemcitabine/paclitaxel group and 2.0 months in the gemcitabine-alone group (HR, 0.64; 95% CI, 0.47–0.89, P = .0067). The objective response rate was 17.1% in the gemcitabine/paclitaxel group and 4.2% in the gemcitabine-alone group (P = .008).
- Third-line therapies were given to 32.1% of patients in the gemcitabine/paclitaxel group and 46.5% of patients in the gemcitabine-alone group.
- The overall incidence of grade 3 or greater treatment-related adverse events was 58% in the gemcitabine/paclitaxel group and 27.1% in the gemcitabine-alone group. In the gemcitabine/paclitaxel group, 13% of patients experienced serious treatment-related adverse events, versus 7.1% of patients in the gemcitabine-alone group. The most common grade 3 or greater treatment-related adverse events among patients in the gemcitabine/paclitaxel group versus those in the gemcitabine-alone group were anemia (15.2% vs. 4.3%), thrombocytopenia (19.6% vs. 4.3%), neutropenia (15.9% vs. 15.7%), peripheral neuropathy (12.3% vs. 0%), and asthenia (10.1% vs. 2.9%). There was one grade 5 adverse event associated with gemcitabine/paclitaxel therapy, which was attributed as acute respiratory distress.
- Based on this study, no OS benefit was seen with second-line gemcitabine/paclitaxel over gemcitabine alone. However, there was an OS benefit in certain subgroups (patients aged 65 years or younger and patients with high CA 19-9 levels at baseline). PFS and objective response rate were significantly higher in the gemcitabine/paclitaxel group. Thus, combination therapy with gemcitabine/paclitaxel can be considered for the appropriate patient, recognizing the higher rate of treatment-related adverse events.
Special considerations for patients with germlineBRCA1/BRCA2variants
Germline variants in BRCA1 or BRCA2 are present in 4% to 8% of patients with pancreatic adenocarcinoma.[
Olaparib
Olaparib (a PARP inhibitor) maintenance therapy can be considered for patients with germline BRCA1/BRCA2 variants and metastatic pancreatic adenocarcinoma who have responded to first-line platinum-based therapy for more than 4 months.
Evidence (olaparib):
- POLO trial (NCT02184195): A multicenter, phase III, randomized, double-blind, placebo-controlled trial that included 154 patients with metastatic pancreatic adenocarcinoma with germline BRCA1 or BRCA2 variants whose disease had not progressed after 16 weeks of first-line platinum-based chemotherapy.[
14 ][Level of evidence B1] The patients were assigned 3:2 to receive olaparib (300 mg twice daily) or placebo and were assessed for PFS.- Of 3,315 patients screened, 247 patients were identified with germline BRCA variants.
- The median PFS was 7.4 months in the olaparib arm and 3.8 months in the placebo arm (HR, 0.53; 95% CI, 0.35‒0.82; P = .004)
- Median OS was 18.9 months in the olaparib group and 18.1 months in the placebo arm (HR, 0.91; 95% CI, 0.56‒1.46; P = .68).
- There were more grade 3 or 4 treatment-related adverse events with olaparib (40%) compared with placebo (23%).
Current Clinical Trials
Use our
References:
- Burris HA, Moore MJ, Andersen J, et al.: Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15 (6): 2403-13, 1997.
- Pelzer U, Kubica K, Stieler J, et al.: A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. [Abstract] J Clin Oncol 26 (Suppl 15): A-4508, 2008.
- Pelzer U, Schwaner I, Stieler J, et al.: Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 47 (11): 1676-81, 2011.
- Conroy T, Desseigne F, Ychou M, et al.: FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364 (19): 1817-25, 2011.
- Wainberg ZA, Melisi D, Macarulla T, et al.: NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet 402 (10409): 1272-1281, 2023.
- Von Hoff DD, Ervin T, Arena FP, et al.: Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369 (18): 1691-703, 2013.
- Moore MJ, Goldstein D, Hamm J, et al.: Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25 (15): 1960-6, 2007.
- Wang-Gillam A, Li CP, Bodoky G, et al.: Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387 (10018): 545-57, 2016.
- Gill S, Ko YJ, Cripps C, et al.: PANCREOX: A Randomized Phase III Study of Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J Clin Oncol 34 (32): 3914-3920, 2016.
- De La Fouchardière C, Malka D, Cropet C, et al.: Gemcitabine and Paclitaxel Versus Gemcitabine Alone After 5-Fluorouracil, Oxaliplatin, and Irinotecan in Metastatic Pancreatic Adenocarcinoma: A Randomized Phase III PRODIGE 65-UCGI 36-GEMPAX UNICANCER Study. J Clin Oncol 42 (9): 1055-1066, 2024.
- Holter S, Borgida A, Dodd A, et al.: Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J Clin Oncol 33 (28): 3124-9, 2015.
- Cancer Genome Atlas Research Network. Electronic address: andrew_aguirre@dfci.harvard.edu, Cancer Genome Atlas Research Network: Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 32 (2): 185-203.e13, 2017.
- Golan T, Kanji ZS, Epelbaum R, et al.: Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 111 (6): 1132-8, 2014.
- Golan T, Hammel P, Reni M, et al.: Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 381 (4): 317-327, 2019.
Palliative Therapy
Palliative therapy options for patients with pancreatic cancer include:
- Palliative surgical bypass procedures such as endoscopic or radiologically placed stents.[
1 ,2 ] - Palliative radiation procedures.
- Pain relief by celiac axis nerve or intrapleural block (percutaneous).[
3 ] - Other palliative medical care alone.
References:
- Sohn TA, Lillemoe KD, Cameron JL, et al.: Surgical palliation of unresectable periampullary adenocarcinoma in the 1990s. J Am Coll Surg 188 (6): 658-66; discussion 666-9, 1999.
- Baron TH: Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 344 (22): 1681-7, 2001.
- Polati E, Finco G, Gottin L, et al.: Prospective randomized double-blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. Br J Surg 85 (2): 199-201, 1998.
Latest Updates to This Summary (02 / 12 / 2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Updated
Revised
This summary is written and maintained by the
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult pancreatic cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Pancreatic Cancer Treatment are:
- Amit Chowdhry, MD, PhD (University of Rochester Medical Center)
- Leon Pappas, MD, PhD (Dana-Farber Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Pancreatic Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at:
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our
Last Revised: 2025-02-12
This information does not replace the advice of a doctor. Ignite Healthwise, LLC, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.
Page Footer
I want to...
Audiences
Secure Member Sites
The Cigna Group Information
Disclaimer
Individual and family medical and dental insurance plans are insured by Cigna Health and Life Insurance Company (CHLIC), Cigna HealthCare of Arizona, Inc., Cigna HealthCare of Illinois, Inc., Cigna HealthCare of Georgia, Inc., Cigna HealthCare of North Carolina, Inc., Cigna HealthCare of South Carolina, Inc., and Cigna HealthCare of Texas, Inc. Group health insurance and health benefit plans are insured or administered by CHLIC, Connecticut General Life Insurance Company (CGLIC), or their affiliates (see
All insurance policies and group benefit plans contain exclusions and limitations. For availability, costs and complete details of coverage, contact a licensed agent or Cigna sales representative. This website is not intended for residents of New Mexico.